NEWS
AND BLOG
The Economic Impact of Counterfeiting: How It Affects Brands and How to Fight Back

Counterfeiting stands for the creation of fake products to sell them as original ones. The economic impact of counterfeiting is massive. OECD estimated global trade in counterfeit goods to be more than $460 billion, which is 2.5% of global trade....
Non-Invasive Brand Protection: Securing Your Products Without Changing Their Look

Non-invasive brand protection is a cutting-edge approach to fighting counterfeits. It works by embedding invisible security features on the product or its packaging. The human eye can't see these microscopic features, but they create a unique, tamper-proof signature that's impossible...
Smartphone Product Authentication: Covert Security Anyone Can Verify

Counterfeit products do more than funnel money away from legitimate businesses. Counterfeiting poses a significant threat to economies, jobs, and consumer safety. It does so by creating an underground economy that skirts regulations and taxation. The problem feels almost invisible...
The Role of Brand Protection Strategies in Preventing Counterfeits

Climate change may be one of the biggest issues the world is facing right now, but so is another crucial thing: counterfeiting. Globally, it has cost businesses all over the world over $500 billion annually, affecting nearly every industry from...
Another Gold Refinery Secured

Over a decade ago, we introduced the AlpVision Fingerprint® technology, which today enables the authentication of hundreds of millions of parts each year across a wide range of industries, including electronic devices, luxury goods, plastic caps, and gold bars. When it comes to gold, our technology allows...
BMS Spring 2025 Release: Total Control and a Seamless Mobile Experience

AlpVision is pleased to announce the BMS Spring 2025 release, delivering major enhancements to security, user management, and mobile usability. A key highlight is the launch of a completely redesigned permission scheme, allowing customers to finely control access and roles for employees,...
Is your brand a target? The growing threat of counterfeit merchandise

Every 10 seconds, a fake product is sold globally, causing financial losses and reputational damage. From luxury items to essentials, no brand is immune to counterfeit merchandise. Counterfeiting thrives across industries, so proactive protection is vital. Solutions like AlpVision lead...
AlpVision to Address Precious Metals Security at IPMI Annual Conference

AlpVision is excited to announce our participation in the International Precious Metals Institute (IPMI) Annual Conference, held from June 7-10 in Scottsdale, Arizona. As the IPMI's premier annual event, it will gather hundreds of professionals from the global precious metals industry, including experts...
AlpVision: Invisible Product Security

The International Chamber of Commerce estimates that counterfeiting costs the global economy over $500 billion annually, threatening brand integrity, consumer safety, and profits. Invisible product security provides a robust solution by integrating covert features that counterfeiters cannot replicate, helping brands...
Counterfeit Perfume Crisis: Protect Your Brand with Cryptoglyph

Counterfeit perfumes are the fourth most seized item by the U.S. Customs and Border Protection (CBP), according to the latest Intellectual Property Rights Seizure Statistics Report. CBP officials seized more than 500,000 fake fragrance items, amounting to over $57 million...
Stop counterfeiting: proven anti-counterfeiting measures for your brand

The global counterfeit market is projected to reach $1.79 trillion by 2030, with severe consequences for brands, including revenue loss and reputation damage. This article explores proven anti-counterfeiting measures to protect your products and customers. The limitations of traditional...
Authentication of Products from Videos and Images

For over 20 years, AlpVision has been delivering applications that integrate both the capture of images with the detection of our covert features. The capture can be done with smartphones, but also with a video camera or scanner, and the detection process can be performed on the...
Tackling the threat of counterfeit airplane parts to ensure aircraft safety

Counterfeit airplane parts pose serious risks to safety, lead to accidents, fines, and legal issues. This article highlights the dangers and helps suppliers ensure part authenticity, protect the aviation industry, and uphold compliance standards. The dangers of counterfeit airplane...
AlpVision Launches AV-Check: A Cutting-Edge Mobile App to Combat Counterfeiting

AlpVision has introduced AV-Check, a new mobile app designed to autonomously protect products against counterfeiting. Developed for partners, printers, molders, prospects, and customers, the app provides a simple and efficient way to verify product authenticity in real time. AV-Check supports AlpVision’s flagship...
Fake brake pads: dangers and solutions

Each year, dozens of families narrowly survive when their car fails to stop due to fake brake pads. Those counterfeits pose life-threatening risks. This article uncovers their dangers and economic impact, as well as solutions to protect brands and drivers....
How counterfeit monitoring can protect your products

Counterfeit monitoring is needed in today’s global market to protect brands and consumers from the dangers of fake products. AlpVision’s Fingerprint technology not only detects counterfeit items but also helps businesses track and monitor counterfeiting activities effectively. Understanding AlpVision’s...
How Cryptoglyph can detect counterfeit cork stoppers and other compressible materials

Counterfeit cork stoppers pose significant risks in the wine industry for brands and consumers alike. Thankfully, AlpVision’s innovative Cryptoglyph technology now extends its anti-counterfeiting capabilities to corks and other compressible materials. Background: Cryptoglyph technology What is Cryptoglyph? Cryptoglyph is...
Microtext: An Outdated Technology

Is your product security stuck in the past? Microtext, or microprinting, was once the go-to solution for embedding tiny, hard-to-replicate text in high-value documents. Today, however, high-resolution scanners and printers have made microtext an unreliable security measure. By the 1990s,...
How to safeguard against the dangers of fake spirits

The rise of counterfeit spirits is an escalating concern, particularly as high-value bottles are prime targets for counterfeiters. Fake spirits pose serious health risks, but fortunately, effective solutions exist to combat this issue and protect consumers. Why Counterfeit Spirits...
How to Identify Counterfeit Footwear

Footwear is one of the top ten seized items by the U.S. Customs and Border Protection (CBP) in the United States, according to their annual report. In 2023, counterfeit footwear worth more than 60 million dollars was seized by CBP,...
AlpVision Confirms Full Compatibility with iPhone 16 Family

Following the release of the iPhone 16 family, including the iPhone 16, iPhone 16 Plus, and iPhone 16 Pro, AlpVision confirms full compatibility with all models. These devices bring substantial upgrades in both photography and processing power. The 8-megapixel iSight...
Upcoming Events

Three important events are coming where AlpVision will be present. We will be at the China International Import Expo event from November 5 to 10 in Shanghai, which was created by President Xi Jinping and is dedicated to foreign companies. It welcomes around...
Detecting counterfeit with a smartphone: the macro lenses revolution

In October 2021, Apple launched the iPhone 13 Pro and Pro Max with a macro lens, a feature kept in later models. This advancement allowed the AlpVision app to check smaller surfaces and to detect counterfeits that couldn’t be checked...
AlpVision Webinar Available Online

Celine and Fred recently held a webinar covering AlpVision's 20 years of innovation in anti-counterfeit technology. The webinar goes through various aspects of deploying AlpVision's security features from a practical standpoint. The following topics are covered: How Cryptoglyph and Fingerprint technologies work The implementation process...
How To Identify Counterfeit Stamps

A recent case of postage forgery revealed that a single person defrauded the U.S. Postal Service (USPS) for over $150 million. This was done by creating fraud postage, from 2019 to 2023. How could such a massive operation go unnoticed...
Counterfeit Medicines: A Global Issue

Life-saving drugs like antibiotics and anti-malaria are some of the most counterfeited products in the world. This issue is not slowing down; medicines continue to be a serious problem across the globe. Pharmaceutical companies need to implement better security measures...
How to Protect Against Lottery Fraud

Lotteries offer the tantalizing possibility of instant wealth, turning everyday life into a potentially transformative experience. Millions of people participate in lotteries, driven by the hope of winning life-changing prizes. However, with lottery fraud on the rise, organizations are met...
Taggants for Brand Protection: What It Is and Why It’s Not Fool-Proof

Counterfeiting affects every industry from pharmaceuticals to luxury goods, and from electronics to consumer goods. Traditionally, companies have relied on taggants for brand protection—special markings embedded into products to verify their authenticity—as a line of defense against counterfeiters. However, they...
Fighting Counterfeit Medical Devices Protects More Than Just Patients

Topical Absorbable Hemostat, a medical device, is commonly used during surgical procedures to control bleeding. Imagine if a surgeon puts the device into a patient's body, but finds out the product isn’t performing well as it’s an instance of...
Why Is Brand Protection Important?

Counterfeiting affects your business in many ways. It harms your brand and your reputation. It also jeopardizes the safety of your customers or patients. Brand protection is important as it allows you to prevent these problems but, depending on your...
Small is Beautiful

As small as 10mm in diameter and still authenticatable with a smartphone, this is what is now possible with the latest evolution of AlpVision Cryptoglyph. This presents a significant challenge for all authentication features, especially for invisible security elements readable...
Honored to Join Industry Leaders: AlpVision at LBMA/LPPM Global Precious Metals Conference 2024 in Miami

We are excited to be attending the upcoming London Bullion Market Association (LBMA) and London Platinum and Palladium Market (LPPM) conference in Miami, FL from October 13-15, 2024. The LBMA promotes good trading practices and ensures integrity in the gold...
Grey Market Diversion: What It Is and How to Prevent It

The grey market diversion is a complex problem for brands to curb. The products are not counterfeited, rather they are diverted to a different location This impacts the brand’s trade partners, pricing strategy, marketing message, financial planning, revenue forecast, and more....
Maximizing Global Engagement: AlpVision’s Strategic Presence Across Social Networks

Social networks have become an integral part of the communication strategies of companies addressing a worldwide market like AlpVision. For several years, we have had a global strategy to be present on the most important networks. For instance, we publish weekly...
HOW TO PROTECT YOUR BRAND FROM COUNTERFEITS

Counterfeit products not only result in lost sales for your business but have the power to destroy your brand reputation and market trust. With such high stakes, brand protection from counterfeiters becomes an essential part of your market strategy, and...
Counterfeit pallets: issues and solution

Counterfeit pallets: issues and solution Pallets play an important role in the logistics and supply chain industry. In response to proliferation of counterfeit pallets, Cryptoglyph technology emerges as a promising solution to authenticate pallets and mitigate the adverse effects of...
2 Factor Authentication for RFID equipped products
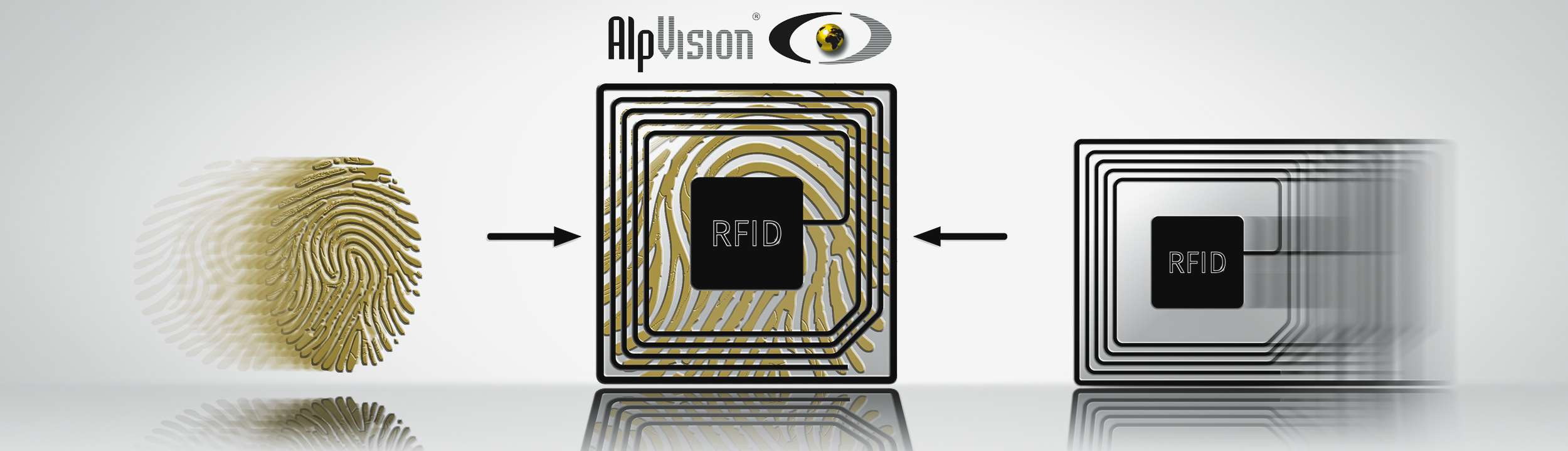
Asking someone about their identity is not enough to tell if the person is lying. In general, an identification document is needed to see if the physical appearance of the person corresponds to the name. We have a similar scenario...
Combat Fake Luxury Pens: How Luxury Brands Can Protect Brand Reputation

Counterfeiters target any and every type of industry, be it a niche or a mass market. Luxury pens fall in the niche category. However, counterfeiters see the market as an opportunity to create fake luxury pens and make exponential profits...
Patent granted to AlpVision

We are happy to announce the granting of a new patent, US11,989,961. It protects a new technology that we have recently implemented in some of our smartphone apps. The main usage of our apps is typically to tell the user if...
Join AlpVision at Virtue Insight’s 14th Annual Pharma Anti-Counterfeiting, Serialization & Supply Chain Security conference in Boston, MA, on June 26 and 27

This event brings together leaders from the pharmaceutical and healthcare supply chain sectors to tackle the global challenge of counterfeit medicines. Key insights will include DSCSA compliance, consumer education, brand protection, smart packaging, government collaboration, and regional market analysis. Lauren,...
Why Mobile Phone Apps to Check Fake Products Is Not the Holy Grail: Brand Owners Need a Comprehensive Strategy

To curb the problem of counterfeiting and to empower consumers against counterfeit items, brands are coming up with smartphone apps to check fake products. The problem is: using smartphone apps without a comprehensive strategy for anti-counterfeiting isn’t helpful. The apps...
Falsified medical devices: risks and solutions

The proliferation of falsified medical devices poses a significant challenge to global healthcare systems. It threatens patient safety and undermines trust in medical products. Some solutions to prevent the risks linked to medical devices counterfeiting exist. The prevalence...
How to prevent food fraud effectively

How to prevent food fraud with anti-counterfeiting technologies Food fraud poses serious risks to consumer health and industry integrity. In this article, we explore innovative anti-counterfeiting technologies designed to safeguard the food supply chain and restore consumer trust. Here...
Consumers May Receive A Penalty For Buying Counterfeit Goods

Some people knowingly buy counterfeit products as a way to save money. On the other hand, some unsuspecting consumers become victims of buying a knockoff unknowingly. In both cases, however, the harmful effects and consumers’ penalties for buying counterfeit goods...
Protection of Desiccant Bags

We are proud to announce that we have signed a new customer for several billion items per year. This company manufactures desiccant bags, which are often counterfeited. These desiccants are added by brand owners to the products they ship to...
Unique Fingerprint for Jewelry

A brand owner in the luxury industry has successfully validated our new, unique fingerprint technology approach. This approach enables the real-time identification of metallic objects using only a smartphone camera and our dedicated app, without any modification of the object....
How do you identify counterfeit goods?

Counterfeit goods detection: tools and tips Counterfeit goods are on the rise, causing substantial financial losses and jeopardizing brands’ reputation. This article explores this growing threat and reveals the importance of some innovative solutions to detect counterfeits and protect...
AlpVision Essentials Webinar

Join us for the inaugural session of our "AlpVision Essentials" webinar series, centered on crucial brand protection issues. Our opening session, titled "Navigating the EU’s Digital Product Passport (DPP)," aims to prepare brands for the upcoming EU mandates on digital...
Counterfeit desiccants – don’t let them drown you.

Desiccants play a pivotal role in maintaining the dryness needed within packaging to ensure the integrity of a wide range of products in industries including food, pharmaceuticals, electronics, and more. These crucial substances are key to avoiding the risks of...
The Hidden Threat: Counterfeit Network Hardware and How to Protect Your Network
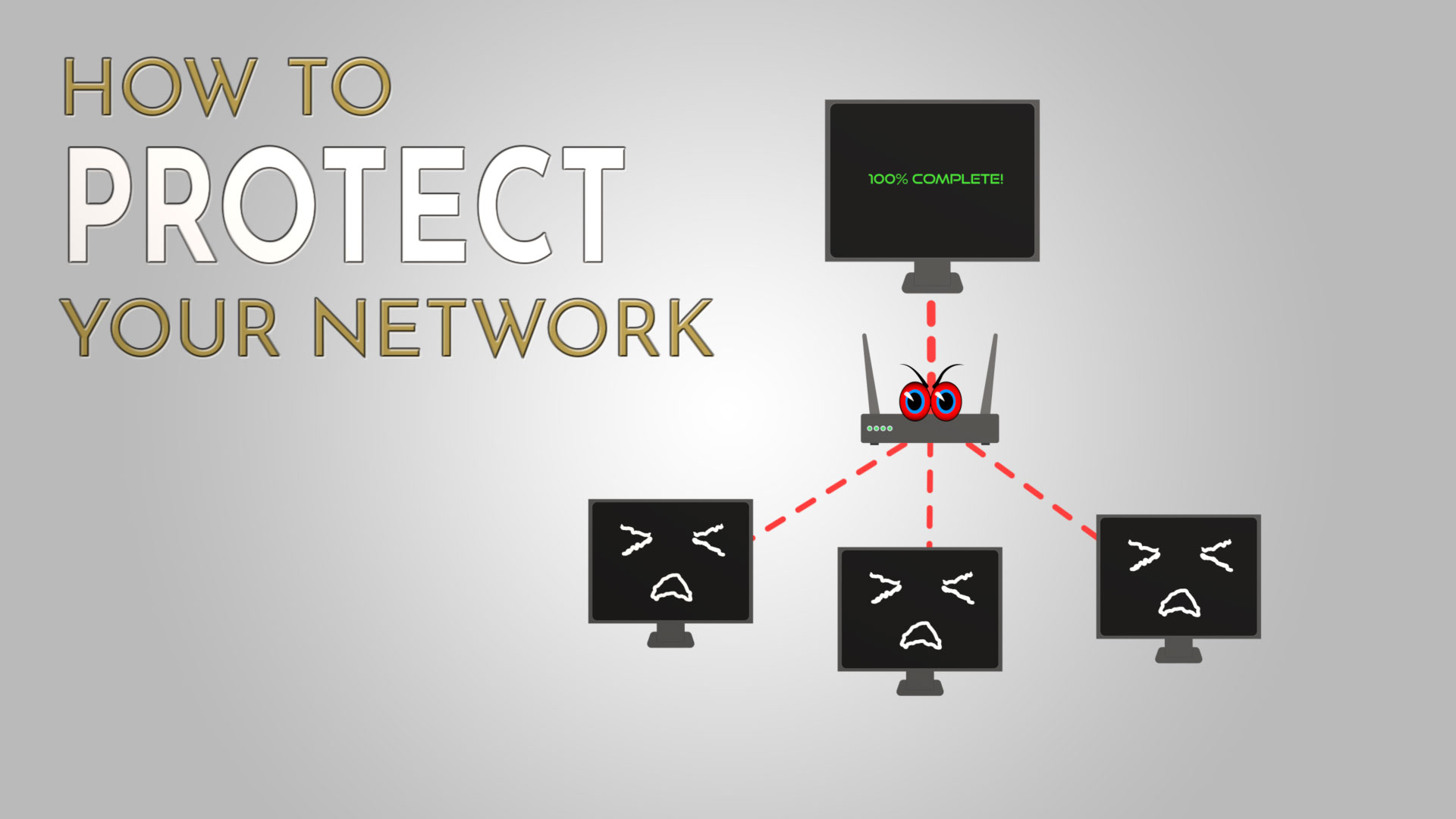
Growing a company in a global environment involves being able to rely on efficient and safe network solutions. The devices set up for a business must fulfill all technical requirements to secure data. This flourishing network gear market is now...
IACC Membership

We are thrilled to share that AlpVision is now a member of the International Anti-Counterfeiting Coalition (IACC), a global non-profit dedicated to combating counterfeiting and intellectual property theft. Since its founding in 1979, the IACC has been actively involved in...
How cork solutions can help fight wine fraud

In the wine world, fraud threatens trust and authenticity. Addressing this is crucial to preserve industry integrity. To fight wine counterfeit, cork is a key weapon, sealing bottles to ensure authenticity and protecting the tradition from deceit. What...
Enhancing the Security of AlpVision’s Fingerprint Technology

Authentication using human fingerprints has been prevalent for years, and the technology for fingerprint readers has evolved significantly to enhance security. A similar evolution is observable in the realm of product fingerprints. Modern technologies are not only based on contrast...
Why is Traceability Important

Government rules and regulations over the world make it necessary for industries like pharmaceuticals and (F&B) to track and trace their products. However, the need for traceability goes beyond a handful of industries. But why is traceability so important that...
Welcoming Honorable US Ambassador Miller

Just after world leaders met at the WEF in Davos, it was both a great pleasure and an honor for AlpVision to welcome the Honorable US Ambassador to Switzerland, Scott Miller, on January 25, 2024. Following a tour of our...
RFID Anti-Counterfeiting Solutions: How to Secure Your RFID Tags

Radio Frequency Identification Technology (RFID) is a cost-effective method to track and identify items, applied in a wide range of industries. On a number of occasions, it’s also used as an anti-counterfeiting tool, which can prove to be ineffective. This...
NFC product authentication – is it really the way to go?

Most corporations are digital friendly because they need a secure process to protect their goods. NFC is a technology used in smartphones, laptops and the fashion industry. So, what is NFC? Who created this electronic innovation, and is it reliable...
Macro Lenses

In October 2021, the first Apple smartphones integrating a macro lens, the iPhone 13 Pro and Pro Max, were launched. Since then, this feature has been retained in iPhones 14 and 15. Although this new feature might appear to many...
Everything you need to know about anti-counterfeit labels

In the realm of product security, the use of anti-counterfeit labels emerges as a pivotal strategy. These specialized adhesive tags, designed for both visible deterrence and covert authentication, play a crucial role in safeguarding products across industries. This article delves...
Spirits Market

Spirits are a market for anti-counterfeiting solutions because those bottles can be expensive, and counterfeits are more likely to be dangerous for consumers than wine. Indeed, spirits have a much higher percentage of alcohol, and when counterfeiters use methanol instead...
Drug Counterfeiting – Why Is It Such a Big Problem?
The consequences of drug counterfeiting are far-reaching and worldwide. According to a review report by United Nations Office on Drugs and Crime, it’s estimated that the black market for counterfeiting exceeds $250 billion. Such a huge supply of illicit medications...
New Partnership with Moonhill Technologies

AlpVision is pleased to announce its cooperation with Moonhill Technologies Ltd, represented by Mr. Sass Farahzad, in promoting the Minting authentication solution in Turkey. Mr. Farahzad's impressive experience in the precious metals field, combined with AlpVision’s leading digital authentication solution...
Growing QR Code Security Risks: How to Mitigate Them

QR codes were first invented to be used for inventory tracking. As they’re extremely easy to use, their usage became widespread across industries like advertising, product authentication, and more. However, with the growing popularity comes the rise of malicious intent...
Combating Counterfeit Seeds: Empowering Farmers for Sustainable Agriculture

Seeds are the root of the global agricultural industry, which is a multi-trillion-dollar industry. This massive scale of the industry makes it a lucrative sector for counterfeiters. These counterfeit seeds have far-reaching consequences on the farmers and the environment. It’s...
Counterfeit Cosmetics Can Damage Brand Reputation More than Grey Market

Counterfeit cosmetics are just as dangerous as pharmaceutical or food counterfeits. It’s a health hazard, and it directly impacts the bottom line of cosmetic brands. In 2020, cosmetic companies suffered a loss of 4.7 billion euros or USD 5.16 billion...
How to Identify Fake Bearings Before Rolling into Death

Bearings are one of the most crucial components in any machinery – from cars to marine ships, and from escalators to a petrochemical plant. This creates a huge market for counterfeit bearings, with the supply of bearings of different shapes...
Managing Halftoning

Since the invention of printing, halftoning has been used to simulate continuous-tone imagery through the use of dots, varying either in size or in spacing, thus generating a gradient-like effect. The first halftone printed image dates back to 1869. Although...
Variable Cryptoglyph

We are proud to announce that, just last month, we launched our first large-scale pilot of the variable Cryptoglyph. This innovative solution embeds invisible serialization data onto packages. In practice, it allows for the restoration of data that gets lost...
Welcoming Lauren to Our AlpVision Team

We are thrilled to introduce you to the newest member of our AlpVision family, Lauren Moise. Lauren has joined us as the Head of US Sales, Marketing, and Operations, and we couldn't be more excited to have her on board....
How Can You Spot Counterfeit Coffee?

Coffee is one of the top ten adulterated and counterfeited food items because of its high consumption worldwide. Billions of dollars are lost in sales for brands every year due to counterfeit coffee. Contaminated coffee is also a health hazard...
The Ultimate Solutions to Tackle Counterfeit Gold Coins

The issue of counterfeit gold coin minting has existed for years. Flooding into the marketplace, these coins pose a huge problem for the global economy. However, minting companies can counter this problem by investing in simple, yet advanced, anti-counterfeiting solutions....
Overview of FMCG Counterfeiting

FMCG products, along with pharmaceuticals and tobacco, are one of the most affected industries by counterfeiting. Investment in covert and overt anti-counterfeiting measures can help brands make life harder for counterfeiters and recover losses in sales and brand reputation.
Counterfeit surgical masks: instant verification is possible

The COVID-19 pandemic kickstarted an explosion in the demand for surgical masks which resulted in crippling shortages. Gripped with fear, people hoarded what they could find and even started stockpiling N95 masks that should ideally have been reserved for use...
Over 1.5M App Uses & 5M Authentications

Putting authentication into the hands of end-users can be both advantageous and challenging. Most product authentication solutions require sophisticated methods and either an expert eye or a trained user to be effective. This remains true even with the advent of...
What is Brand Protection? A definition

Despite widespread awareness, many companies still don’t have a robust offensive against brand abuse. Brand protection is more crucial than ever to safeguard brand identity and should be an integral part of a brand’s overall anti-counterfeiting strategy. This raises the...
New technology for compressible materials

Historically, our invisible marking technology, the Cryptoglyph, has mostly been printed on rigid materials. The only exceptions are cases when it is printed on plastic films. But even on deformable films, the distance between the Cryptoglyph dots is invariant. We...
New Patent: Authenticating 3D Structures

A new European patent, EP 3 374 975 B1, protects a method for authenticating a 3D structure by scanning it with a 2D image. Developed by AlpVision and marketed as SmartEmbossing, this technology allows three-dimensional structures to be authenticated using...
What Is Serialization in Pharma and Why It Can’t Replace Verification

Pharma serialization is indispensable to the fight against counterfeit drugs but without advanced verification measures, it’ll continue to remain only a blunt tool. Covert counterfeit protection will only compound serialization’s significant benefits.
Counterfeit Pet Food – Protect Your Brand From This Plague

Thousands of pets have already succumbed to the plague of fake pet food. With the authorities unable to catch every single culprit, the onus lies on brand owners to insist on covert solutions to achieve counterfeiting protection that’s completely tamper-proof.
How to Spot a Fake Designer Handbag

While cheaper bags are a smaller upfront investment, you'll typically pay more in the long run. Designer bags are sturdier and last longer, but you need to be careful; the market is saturated with fakes. Let’s explore how to spot...
Luxury Transparency Index

The Luxury Transparency Index is a public-private partnership led by the Swiss Federal Institute of Technology (EPFL) and Lausanne University (UNIL) with a primary focus on promoting transparency within the luxury sector. Its main objective is to assess the social...
Authentication of Plastic Bags

We are delighted to announce that we have secured a new customer in Asia! The requirements for this project were particularly demanding. Specifically, our task was to protect a small, flexible, transparent plastic pouch that contains a powder. Due to...
Veriscan: 10 Years of Shining Success in Authenticating Precious Metals

Authentication of precious metal products with a smartphone was once deemed impossible until AlpVision proved otherwise seven years ago. In 2006, AlpVision patented the AlpVision Fingerprint technology, which has since evolved to a level where it can be utilized by...
Real or Fake Bourbon: Here’s How to Spot Fake Bourbon

How to spot fake bourbon? The question reveals an endemic problem affecting the biggest brands in the whiskey industry. Brands need to explore next-generation technology solutions to take back control from counterfeiters.
How do I make my QR code secure?

QR codes have many use cases, yet there's a problem with deploying them for anti-counterfeit security. You must use QR codes as intended, or con artists will circumvent them. So, brand owners often ask, how do I make my QR...
What Happens If You Buy Counterfeit Goods?

Buying counterfeit products may seem like a victimless crime, but in reality, the consequences are far-ranging and widespread. You may not know that counterfeiting enables illicit activity worldwide, so what happens if you buy counterfeit goods online?
Meet us at the Global Brand Protection Innovation Programme

AlpVision is excited to announce that we will be participating in the 17th Global Brand Protection Innovation Programme, taking place on the 24th and 25th of May, 2023 in Frankfurt. As a leading provider of digital authentication solutions, we are...
AlpVision Cryptoglyph integrated into WeChat

“WeChat” is the most used smartphone app in China, with about 1.3 billion users. It was initially a simple app that facilitated people to chat with each other. However, it has now become an app that can be used for...
LBMA Accreditation

AlpVision has been accredited by the LBMA Gold Bar Integrity (GBI), an international independent authority for precious metals. This accreditation confirms the cutting-edge anti-counterfeiting technologies of AlpVision, allowing us to partner with major players across the global precious metals industry....
How Much Money Do Brands Lose to the Counterfeit Fashion Industry?

The lucrative counterfeit fashion industry costs fashion brands billions every year. Brands have virtually been unable to make a significant dent in this illicit global trade. With counterfeiters getting bolder on social media, is there hope for manufacturers? Advanced anti-counterfeiting...
Physical NFT: Can It Be Used to Fight Fake Products?

Blockchain-based digital assets, like non-fungible tokens (NFT), bring new opportunities for your brand, but you must put this evolving tech in context to yield maximum value. Otherwise, you risk investing in a physical NFT that doesn't safeguard products.
Counterfeit Viagra and Other Counterfeit Erectile Dysfunction Drugs: Solutions to Protect Patients

Counterfeit Viagra and other medications to treat erectile dysfunction (ED) pose a severe threat to public health and safety. Although buying these pills online may be legal, consuming them is dangerous for patients looking for affordable medicines.
Why luxury’s counterfeit problem is getting worse

Today’s anti-counterfeiting technology provides brand owners with state-of-the-art protections, yet fraudsters continue to flood luxury markets with fake products. The challenge is understanding why luxury’s counterfeit problem is getting worse.
How to Protect Against Counterfeit Food

When consumers unwittingly consume counterfeit food, the consequences can be life-threatening. Fake food still reaches store shelves despite a company’s best efforts to manufacture safe products, so the need for better anti-counterfeit solutions is imperative.
Precious Metals and Anti-counterfeiting Technologies

The protections are diverse and sophisticated when it comes to anti-counterfeiting technologies for precious metal mints. The stamped engravings on the surface of gold kilobars and bullion can prove their authenticity, but they can also be faked.
Does Blockchain Provide Authenticity of Physical Products?

Bitcoin’s rapid expansion over the past decade has propelled blockchain technology to new heights. Today, the tech is still maturing, and companies continue to experiment with other use cases. So, does blockchain provide authenticity of physical products too?
How to Spot a Fake Fire Extinguisher

The consequences of using a fake fire extinguisher can be deadly. A fake device will malfunction, and it may also intensify the fire. Indeed, the situation warrants high-tech solutions because the cost of failure couldn’t be higher – injuries and...
PATENT GRANTED ON MACHINE LEARNING APPLIED TO AUTHENTICATION
On October 4th, 2022, AlpVision was granted a 24 page US patent. This invention allows for the combination of machine learning and AlpVision technologies. Our current detection algorithms are composed of several processing blocks, each with specific parameters. Using...
SECURITY FEATURE SELECTION TOOL

Implementing an anti-counterfeiting security feature, as a brand owner, is not an easy task. Aside from strategic and financial considerations, selecting the most appropriate anti-counterfeiting technology is critical. Deploying a technology is a long-term commitment that affects not only the...
How Can You Tell a Fake Circuit Breaker

When electricians inadvertently install fake circuit breakers, the consequences can be severe. Not only can counterfeit breakers cause fires, but they can also lead to life-threatening injuries or death. Still, brands can protect consumers with new tech.
How to Identify Fake Engine Oil

Counterfeiters and fraudsters will stop at nothing to ply their criminal trade to turn a profit. As such, fake motor oil is a persistent threat that demands a better solution, especially as criminals try to take advantage of fluctuating global...
How to Spot Fake Baby Formula

Counterfeiters will try to fake any product, including baby formulas. The moral revulsion against potentially harming infants isn't there. Instead, fraudsters only care about profits, but brand owners are not helpless.
BMS OPENS UP TO LINUX
In a continuous effort to adapt our anti-counterfeiting solutions to customers’ requirements, and not vice versa, we are extremely pleased to report that AlpVision BMS – Brand Monitoring System – is now capable of running on Linux Servers. BMS is AlpVision’s anti-counterfeiting monitoring and...
Counterfeit Doping Control Kit – Can We Avoid This?
Competitive sports aren’t immune from counterfeiting despite the best efforts of organizations worldwide. The need to identify a counterfeit doping control kit makes the challenge more complex. Still, brands have options regarding anti-counterfeit protection.
THE RACE BETWEEN SMARTPHONES AND GROWING COMPUTATIONAL NEEDS
All our customers use our smartphone apps for authenticating products. Their needs are constantly growing: we went from a few million items per year in 2000 to hundreds of millions in 2010 and reach now tens of billions of products...
AUTHENTICATION OF AUTOMOTIVE LUBRICANTS

AlpVision is the authentication feature supplier protecting the largest number of products in the tobacco, pharmaceutical and precious metal markets with digital covert solutions. Moreover, we have the leadership in those industries for the past 15 years. Interestingly, we recently...
How to Identify Fake Products Using Barcodes – Is It Possible?

Counterfeiting can occur at any point in the product life cycle, whether it originates at the manufacturer or as distributors unwittingly transport fake goods. One strategy to pinpoint counterfeits is to identify fake products using barcodes, but do they work?
What are invisible QR codes and how do you secure them?

QR and barcodes are used for traceability. However, when products are distributed on the gray market, these codes are often erased, thus defeating their purpose. Using secured invisible QR codes can solve this problem.
Security and taggant inks: why they aren’t suitable for brand protection

Banknotes, passports, certificates, checks, identity cards; security inks are found in all of these items and more. They support authentication and reduce the risks of tampering and counterfeiting. Yet when it comes to brand protection where brands need to protect...
LOOKING BACK AT 12 YEARS OF ALPVISION FINGERPRINT

AlpVision Fingerprint is a technology that uses the matte finish of plastic and metal parts as an authentication feature. From a mathematical perspective, the detection is somewhat similar to the Cryptoglyph, but it also has a unique feature: It requires...
How to Protect Against Counterfeit Batteries
Counterfeiting is a multi-billion dollar criminal trade, and the perpetrators will try to fabricate facsimiles of just about everything, including counterfeit batteries. As such, the need for brand owners to protect against counterfeit batteries is clear.
AlpVision’s patent uses artificial intelligence for product authentication

Artificial intelligence (AI) is currently a very hot topic and has applications in many different fields. AlpVision has already been using AI for many years in some of its smartphone apps in order to increase the detection performance of genuine...
Counterfeit Casino Chips – How to Spot Them Easily

Regarding the gaming industry, counterfeiting has remained a persistent problem since its inception, but why? In short, fraudsters and con artists have all the incentives they need to ply their trade: gambling winnings with no actual financial risk.
Pharma Traceability Solutions
Pharmaceutical companies regularly face problems relating to counterfeiting. One way to solve this issue could be an efficient pharma traceability solution. But what is pharma traceability in the first place? Why ensure such measures for counterfeiting protection? Read on to...
What Is Microprinting, and Why Is It Outdated?

The evolution of microprinting as anti-counterfeit protection was a boon for brand owners worldwide since the printing technique was initially hard to replicate. Few had access to the technology required to create it, but what is microprinting exactly?
How Do You Prevent Counterfeiting?

As a brand owner, it's best to accept that counterfeiting remains a severe problem despite increased awareness. Intellectual property laws and trademark infringement protections are helpful, but how do you prevent counterfeiting in the first place?
Anti-counterfeit technology: from invisible ink to smartphone apps

The evolution of anti-counterfeit technology has progressed over the last two centuries in ways no one ever imagined. Indeed, counterfeiting itself has a lengthy history, so anti-counterfeit protections developed long ago to secure goods as well as currency.
MONITOR SPECIFIC COUNTERFEITERS PRODUCTS WITH OUR FINGERPRINT TECHNOLOGY
AlpVision Fingerprint enables authentication of plastic molded products by identifying microscopic details of the plastic microstructure. The big advantage of this approach is that it requires absolutely no modification of the product itself. A customer asked us recently to apply...
Apple Ipod Touch is history

After 20 years of existence, Apple has decided to go “end of life” with their iconic iPod. The original iPod was introduced as a portable music player back in 2001. From there, it has undergone an amazing evolution. Starting with...
What is product authentication technology?

Companies have many options when it comes to product authentication technology, yet some haven’t picked a solution – or even decided on an anti-counterfeit strategy at all.
What is anti-counterfeiting?

Counterfeiters are persistent, sophisticated, and determined criminals. As such, brand owners would benefit from a comprehensive anti-counterfeit strategy. But you can safeguard your brand in many ways, so what is anti-counterfeiting? What does it include?
Brand Protection – Hologram: Why Is This Technology Outdated?

Any form of brand protection - hologram or not – depends on developing a holistic anti-counterfeit strategy, including the underlying IT systems that your company already has in place. The challenge is turning away from the usual slate of visible...
VIDEOS ABOUT ANTI-COUNTERFEITING TECHNOLOGIES

AlpVision has been providing anti-counterfeiting features (ACFs) to brand owners for over 20 years. In this process, we have developed a unique, wide-ranging expertise. Therefore, we decided to share this knowledge in short videos. Each one-minute video is dedicated to...
What is product protection?

There are plenty of reasons why companies need anti-counterfeit technology to protect their brand, so the primary challenge is deciding on the correct type of product protection because not every software includes similar features and integration capabilities.
LET THE NUMBERS SPEAK

Regular readers of the newsletter may recall that AlpVision solutions protect over 30 billion products a year. Until a couple of years ago, our solutions were exclusively reserved for internal use by brand owners. However, with end users and consumers...
Can QR Codes Be Counterfeited?

One of the most common questions we answer at AlpVision is this: can QR codes be counterfeited too? The short answer is that QR codes are counterfeit-able, but you have additional options to secure them. The challenge is determining which...
What are anti-counterfeit solutions?

When you need to protect your brand, you have several options for anti-counterfeit solutions. But since not every anti-counterfeit technology is as effective as you'd expect, we'll outline the essentials in this article to provide critical context.
Why Are Security Labels Not Secure?

Security labels used to have an advantage over other counterfeit protection, but you should know that fraudsters have finally caught up to the technology. Now, it’s more critical than ever to develop a new approach to protect your brand.
Major progress on invisible serialization with Cryptoglyph
The Cryptoglyph is an invisible feature which protects over 30 billion of products every year. A positive detection using our smartphone app proves that the product is genuine. However, some of our customers need something fundamentally different: a feature that...
Looking back to year 2021

2021 was an historical date for AlpVision which celebrated its 20th anniversary. But it was also a year of commercial extension with an important agent contract signed in China and the integration of two organizations: Unifab in France and Stop...
Cannabis Anti-counterfeit Solution: How to Perform CBD Authentication

The cannabis industry presents new business opportunities. Unfortunately, it also opens the door to counterfeiters trying to take advantage of the momentum. Without a doubt, fraudsters have every incentive they need, so how do you plan to safeguard your brand?
AlpVision and PharmaLedger

Following PharmaLedger’s year 2 press release in December 2021, we are happy to share our contribution to the European Project. Sponsored under the Horizon 2020 program, PharmaLedger brings together 12 global pharmaceutical companies and 17 public and private entities. The...
How to spot a fake collectible card like a pro and what can you do to stop it

Security And Trading Cards How to spot a fake collectible card has become a primary topic of interest in the TCG (Trading Card Game) community. After all, the collectible card industry has set its roots as a valuable market. Logan...
Protect Your Brand with a Watch Certificate of Authenticity

The benefits of a watch certificate of authenticity are many, yet many brands still struggle to keep counterfeits out of circulation. Indeed, it’s always been a concern in the watchmaking industry for as long as we’ve been manufacturing them.
Here’s Why Counterfeit Goods Are Bad and Harder to Prevent

Counterfeiting isn’t a victimless crime since it’s so prevalent worldwide, but do you understand why counterfeit goods are bad? Who is paying the price - sometimes literally - for the rampant counterfeiting of consumer goods, pharmaceuticals, and food?
Enhance Authenticity with BMS based Product Serialization

Pharmaceutical companies and businesses often need to track packages through the industry’s supply chain. Companies need to trace their products at an individual and pallet level to ensure that clients receive the right products. Product manufacturing businesses also grapple a...
Is Optically Variable Ink (OVI) Secure?

Counterfeiting is a massive problem. To help secure valuable items such as banknotes, the most common method today is through optically variable ink (OVI). In a nutshell, OVI ink appears to change colors based on the angle of viewing. How...
Do You Know How to Spot Fake Supplements?
With so many counterfeit nutritional supplements available, it has become more challenging for brands to gain consumers’ trust with legitimate supplements containing safe, quality ingredients. So, the question is this: do you know how to spot fake supplements?
ALPVISION SOLUTIONS RELATED TO COVID-19
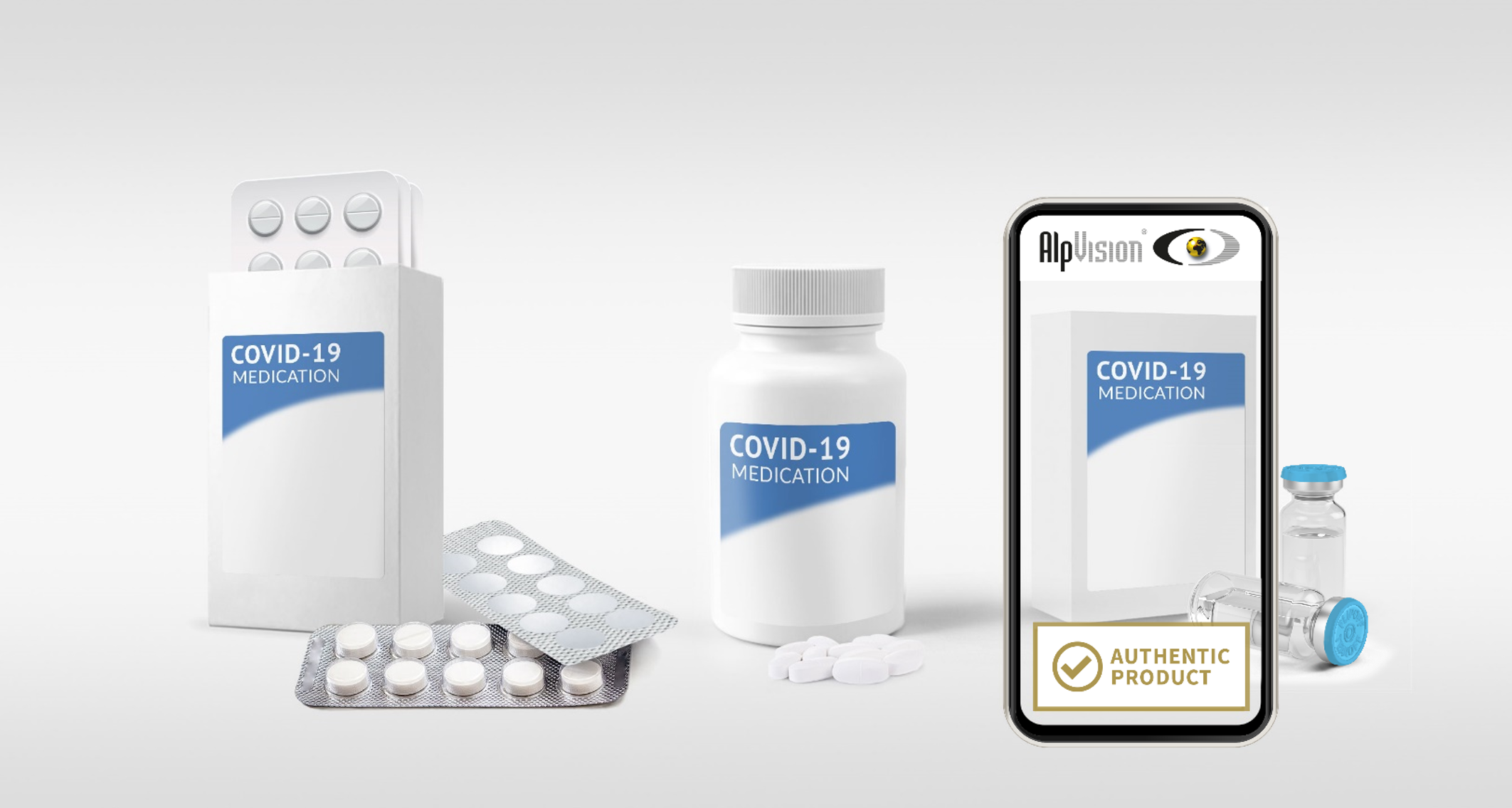
With our solutions, AlpVision is actively contributing to solving the counterfeiting problem that has occurred with COVID-19-related pharmaceutical products. Last year, we launched the COVID-19 Initiative, offering special conditions for protecting against any counterfeit drugs related to COVID-19. This offer...
Stop Piracy Member

As of September 10, 2021, AlpVision is a proud member of the Swiss non-profit association Stop Piracy. The association provides educational and awareness campaigns to consumers and is very active in cooperation between the authorities and businesses. The association’s work...
How to Secure QR Code? Combine it with a Covert Security Feature!

QR codes have become more popular than ever. However, one needs to keep in mind that QR codes have been designed for traceability and not authentication. So, how do you secure QR code? Fortunately, this is possible and we show...
How to authenticate shoes: Technical solutions for brand owners

Shoes are the most counterfeited item globally, and the fraudulent industry only seems to be growing in size. Brands need to protect themselves by harnessing better solutions to authenticate shoes.
Covert security features – the choice is not only based on security

Covert security features are among the most secure features. However other factors must be considered while choosing the most appropriate features such as target audience, deployment complexity and cost. Discover below the most well-known features and compare them to your...
NEW VIDEO: OUR SOLUTIONS IN 2 MINUTES!

We finally published our new video, and it looks good! For the first time, we have used graphical illustrations to explain our two core technologies. This is particularly challenging since both technologies are fundamentally covert. For this reason, we have...
ALPVISION ISO 9001-2015 RE-AUDIT IS A SUCCESS

We are proud to report that AlpVision has successfully passed the ISO 9001-2015 re-audit. The ISO 9001:2015 standard, issued by the International Organization for Standardization (ISO), specifies requirements for a quality management system within organizations. Obtaining and maintaining the certification...
Counterfeit Pet Medications Are Out There: Here’s How to Avoid Them

When people think of counterfeit products, they usually think of knockoff designer items and electronics, not pet medications. The truth is, counterfeit pet medications are a growing problem, and they pose a real danger to our beloved animals. Here’s what...
NEWS FROM CHINA

Following are two news item directly from our office in Shanghai, China: The China National Intellectual Property Administration has granted AlpVision's patent application. This solution is a visible 3D authentication solution which is very inexpensive to manufacture, extremely hard to...
Counterfeit Detection Devices: A Panorama
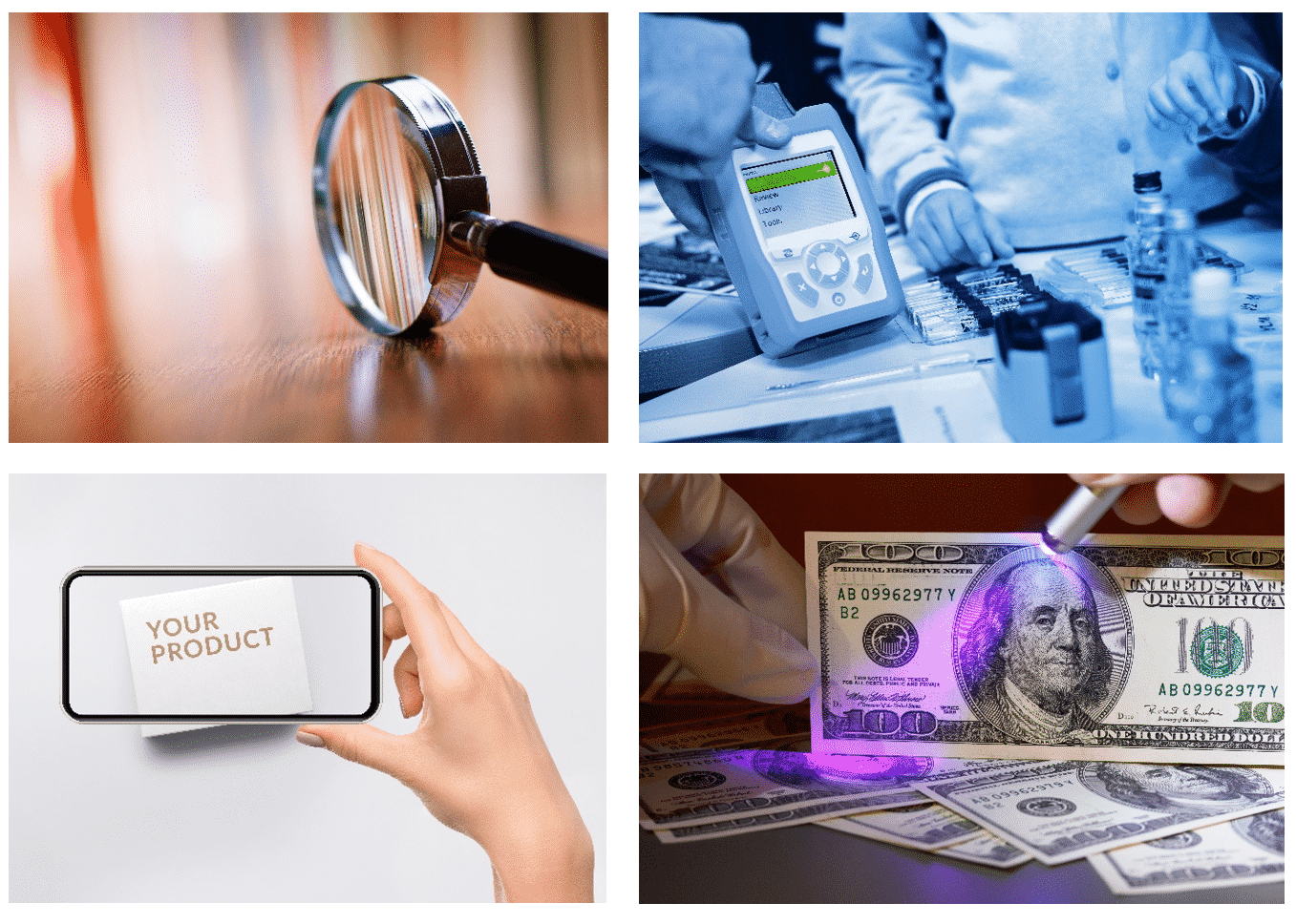
Counterfeit detection devices are devices that are used to prevent or mitigate the creation of counterfeit versions of a product. There are different kinds of counterfeit detection devices, each with their own advantages and disadvantages.
Counterfeit Electronic Cigarettes Are Everywhere. Here’s How to Fight This Problem

With the growing popularity of e-cigarettes, vape pens, and other electronic nicotine delivery systems, the market for alternatives to traditional cigarettes is booming. At the same time, the marketplace is also experiencing an influx of dangerous counterfeit electronic cigarettes.
YET ANOTHER PATENT FOR ALPVISION
A new patent application was granted on March 30th, 2021. It is a so-called “Continuation” of our US Fingerprint patent which refines the claims of this important patent, hence enforcing our protection in the US. This process requires the US...
Fighting counterfeit medical devices protects more than just patients

There is a big problem with counterfeit medical devices. Although the protection of patient safety is the main concern, any precautions taken indirectly protect health professionals in case of a problem. At AlpVision, we have a solution to verify the...
TOBACCO ASIA TALKS ABOUT ALPVISION

In their April 2021 issue, the Tobacco Asia magazine published an article about our authentication solutions. An electronic version is available online here. We are proud to be given this opportunity since Tobacco Asia is the only magazine written and...
ALPVISION CELEBRATES 20 YEARS

This month, we are proud to celebrate the 20th anniversary of AlpVision. AlpVision is a real startup story. From humble beginnings, working from a garage in 2001, AlpVision first started developments of obscure robust steganography technologies. Although these certainly worked great,...
Introduction to ISO 22383 – Selecting authentication solutions

ISO 22383:2020 gives guidelines for performance criteria and an evaluation methodology for authentication solutions. We help you to better understand the key points of the standard towards an insight-driven and well-thought-out choice of your authentication system.
Can counterfeiters imitate covert and overt solutions?

Businesses are now largely implementing anti-counterfeit measures and heavily investing more money in them than at any point in history. Firms are prepared to spend even more on covert and overt solutions than they are today.
Next generation plastic recycling technologies

The future of plastic recycling is in accurate sorting to allow circular plastic economy of such materials as recycled PET, PPT, PP. Plastic is a very useful material, which is hard to replace in many applications. However, the way we...
How can we fight counterfeit plastic objects?

The counterfeiting of plastic objects is a growing problem. More and more companies are falling victim from counterfeit plastic products. AlpVision can help fighting this problem with their patented AlpVision Fingerprint technology.
LASER ENGRAVED CRYPTOGLYPH
Our Cryptoglyph solution is currently printed on 30 billion products every year by over 200 printers worldwide. The integration to the printing process does not add any cost to the production and the solution works on all rotary printing processes...
ANOTHER PATENT GRANTED IN CHINA

On April 8th, we were informed by the China National Intellectual Property Administration that our new patent titled “Method of Authentication of a 3D Structure” had been granted. All 13 claims of our application were accepted by the examiner. This...
PRESENT AT SEVERAL VIRTUAL EVENTS

AlpVision team has recently taken part in various virtual events. Soon, we will present AlpVision’s anti-counterfeiting features during the 2nd Brand Protection V-Congress. This virtual event will be held from the 20th to the 21st of May. Fred Jordan, CEO,...
Fighting drug counterfeiting should not be the job of pharma companies

Pharma companies develop and produce drugs for the good of humanity. But today pharma companies have to also protect their products against counterfeiting. Should this really be part of their job, I don’t think so. A four-pillar approach is proposed...
Should you control your distribution channels with invisible serialization?

This is certain – a reliable serialization technology product tracking is needed. The control of distribution channel is necessary to avoid profit losses and brand reputation damages caused by product diversion. This is not easy, because anyone involved in diverted...
Counterfeit medicines: A global issue

Did you know both antibiotics and anti-malaria treatments are the most counterfeited products in the world? This issue is not slowing down; fake or counterfeit medicines continue to be a serious problem across the globe. As a company committed to...
MEMBER OF UNIFAB LAB

We are proud to have been selected to be a member of the UNIFAB LAB. Known in English as “The Manufacturers Union”, this French association promotes the protection of intellectual property and the fight against counterfeiting at an international level. Their mission statement...
How grey markets and parallel trade can impact your product and what can you do about it?
Control of the distribution channel to prevent product diversion to grey markets and parallel trade is an important priority of brand owners. It is necessary not only to avoid profit loss but also to: protect brand owners from the reputation...
HOW ALPVISION REMEMBERS 2020
2020 was a year in which AlpVision had to explore a whole new set of unexpected and even weird challenges, and all this while doing business as usual. The result of 10 racking months was overall very positive, with new customers, some...
ANOTHER GRANTED PATENT FOR ALPVISION
We are proud to announce that we just had another patent granted. This US patent 15/985,091 is actually protecting our “Smart Embossing®” technology (which is also an AlpVision trademark). This solution is overt and looks a bit like a hologram. However, it is...
For pharma products, only instant authentication and traceability make sense!
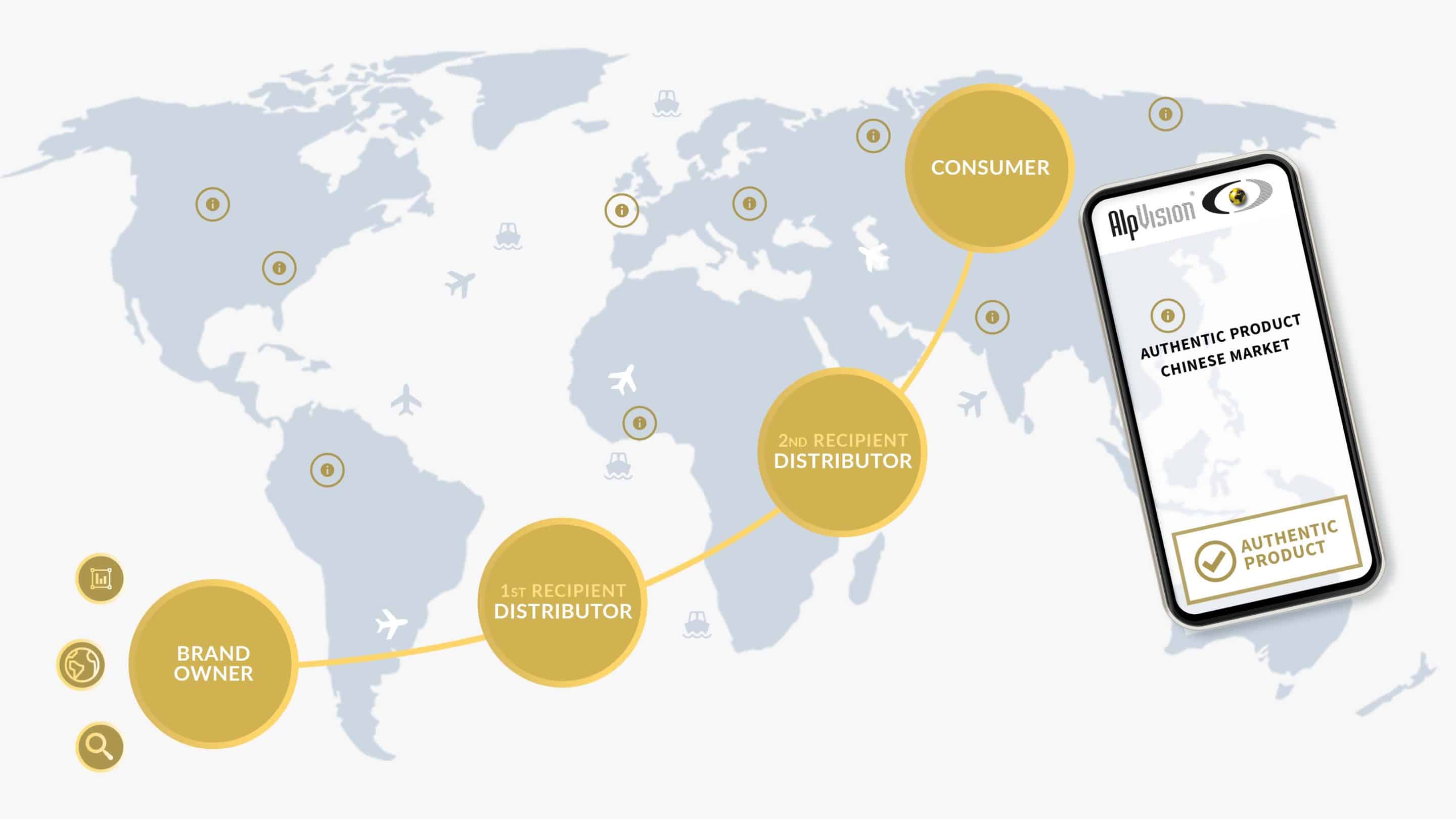
It is a challenge to combine instant authentication and traceability of products for pharma companies. Although traceability is often mandatory it does not protect against counterfeiting. Cryptoglyph combined with any traceability solution will effectively secure the supply chain.
Why you should avoid combining hologram and QR codes for counterfeit protection?
Increasingly, an important aspect of consumer protection is to secure the supply chain by implementing traceability and counterfeit protection. It is important to distinguish between anti-counterfeiting solutions and supply chain technologies. Combining hologram and QR codes will not be the...
INVISIBLE SERIALIZATION USING CRYPTOGLYPH®
The AlpVision Cryptoglyph® technology is printed on over 30 billion product items each year and has been proven to be both easy to use and effective in detecting counterfeit products. However, we have recently met and discussed with companies with a totally...
EVENTS AND ALPVISION IN THE PRESS

AlpVision has recently featured several times in various Swiss news publications such as 24 heures and Le Bilan regarding an authentication solution for precious metals. Additionally, an entire page has been dedicated to precious metals on the AlpVision website. The European Pharmaceutical Manufacturer magazine has also written an...
Free anti-counterfeit solution for COVID-19 medicines
Worldwide, countries are struggling to manage the recurrent waves of COVID-19 infection. In an emergency scenario criminals have more opportunities for counterfeiting, with catastrophic consequences: this is the reason why AlpVision has decided to offer its mobile-based solution (the Cryptoglyph®)...
Why protect against counterfeiting

In this article we will go through the 5 major reasons to protect against counterfeiting despite the fact that anti-counterfeiting solutions will only allow you to spot counterfeits: liability, regulation, reputation, profit and company value.
ASSESSMENT OF ALPVISION FINGERPRINT ROBUSTNESS

The AlpVision Fingerprint technology is an authentication solution that uses the micro-defects naturally occurring during the manufacturing process of products for instance the texture of molded plastic caps. This technology is interesting because it has no impact on the production...
A BETTER WAY TO COMMUNICATE
This year has seen AlpVision develop better modes of communication with our Chinese customers. Since the launch of our new website, a language section is now available in English or Chinese. It is believed that up to 45% of the...
THE WORLD IS TALKING ABOUT US

Two more media articles covered AlpVision solutions during the month of September 2020. The September issue of Bilan, a French language Swiss publication that has been reporting on the Swiss economy for the past 30 years, has published an article...
NEW PATENT FOR ALPVISION
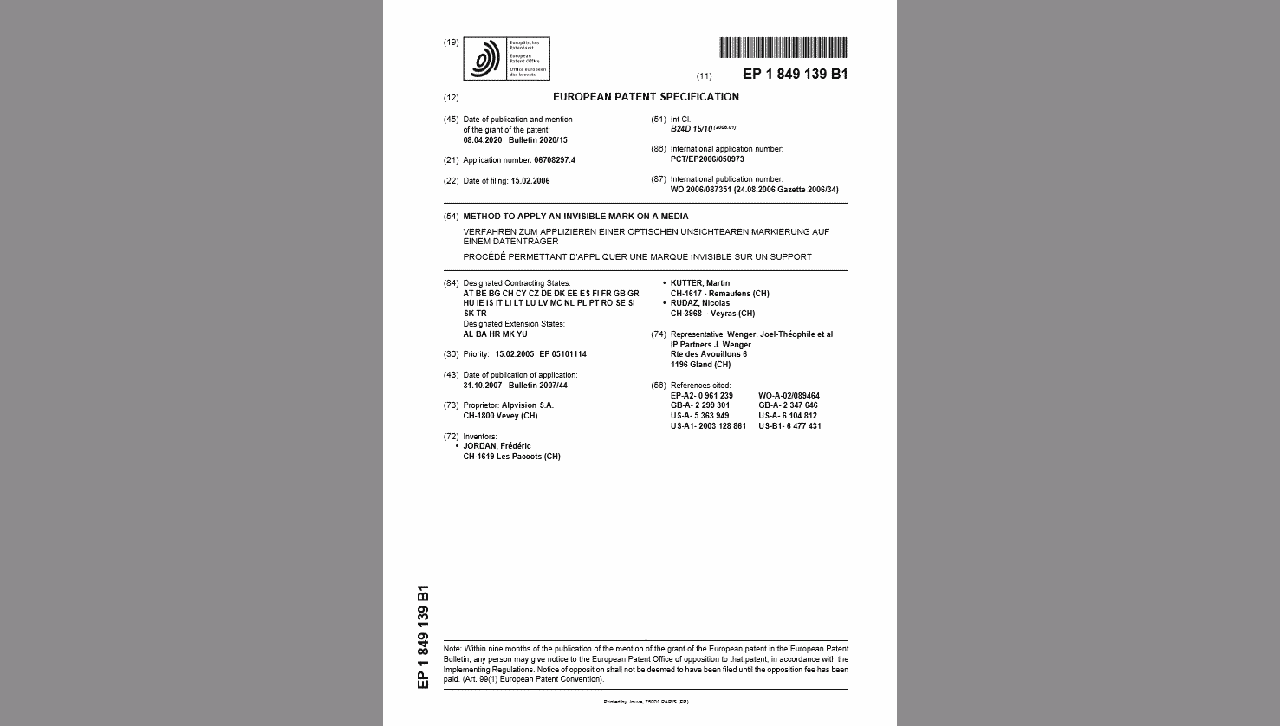
We are happy to announce that we received the official granting of another European patent (EP1849139) titled “Method to apply an invisible mark on a media”. This patent protects the technology we invented for creating and detecting patterns in the varnish...
AlpVision named in the TOP 10 Security Solution Providers

Last June, the magazine Industry Era decided to publish a list of the “10 best security solution providers for 2020”. Obviously, many brand protection suppliers from all over the world submitted applications. We are immensely proud to announce that AlpVision...
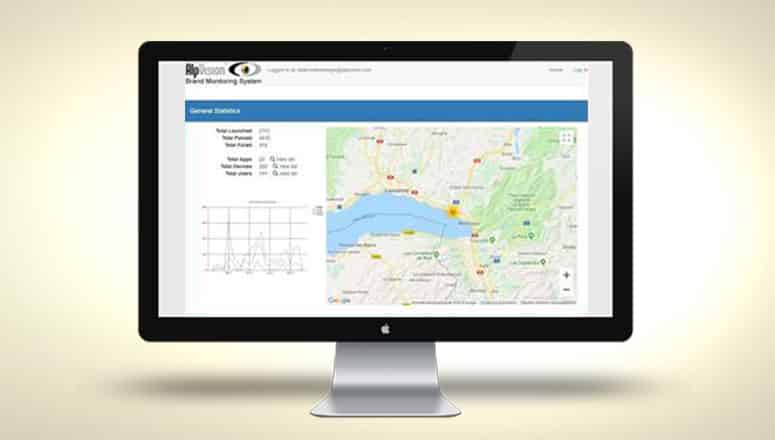
BMS 3.0 – SPRING RELEASE 2020
We are happy to share with you the release of BMS 3.0. With this release, the AlpVision Brand Monitoring System platform has reached an important milestone, providing brand owners and authorities with important insights into brand protection activities and related...
Mobile Product Authentication for Instant Results

Product authentication is a very important subject due to the ever-increasing number of counterfeits today. There are dozens of technologies around, all having different strengths, weaknesses, and usages. In this article I will look at Mobile Product Authentication and highlight...
How to choose a secure blockchain anti-counterfeit solution

Blockchain is becoming more and more popular, tempting companies to replace their current anti-counterfeit solutions with a blockchain approach. Although blockchain has many advantages, including tamper-proof data and perfect traceability of the supply chain, one must be cautious about compromising...
ANOTHER WORLDWIDE DEPLOYMENT

We are in the process of completing another worldwide deployment of the Cryptoglyph technology. This deployment presented a challenge because of the extreme variability of the printing suppliers: from large, sophisticated infrastructures to very small, basic, flexographic presses, all from...
PHARMACEUTICAL COUNTERFEITING “WHO CARES”

Brand protection, counterfeit protection, track and trace, and product authentication are just few words addressing an extraordinarily complex challenge faced by businesses today. According to a 2019 report from the Organisation for Economic Co-operation and Development “OECD”, trade in fake...
SECURING USING LASER ENGRAVING

One of our customers recently came to us with a specific requirement: they wanted to have our Cryptoglyph technology on their products but did not trust their suppliers to do so. At the same time, their bottle filling lines were...
Why is Brand Protection important?

Counterfeiting affects your business in many ways. It can harm your brand and your reputation. It can also jeopardize the safety of your customers or patients. Protecting your brand not only allows you to prevent these problems but, depending on...
An exciting solution for authenticating precious metals

After several years of developments and successful field tests, we are proud to announce the availability of our system for the authentication of precious metals. This technology enables to automatically check if a gold ingot or coin is genuine. It...
AlpVision Fingerprint Patent

On June 25th, 2019, AlpVision was granted one more patent in the United States. The patent with number US 10,332,247 B2 is entitled “Means for using microstructure of materials surface to protect as a unique identifier” completes our patent portfolio...
COVID-19: AlpVision is up and running

COVID-19 is currently impacting worldwide business activities. We have entirely restructured our activities organization in order to handle this exceptional situation, in particular using extensive use of remote work. Surprisingly, this does not seem to substantially impact our productivity overall....
The Success of Innovation

During the last 18 years, we have secured billions of products using our Cryptoglyph technology. In the beginning, the solution worked only on paper. Over the years, we found solutions to enable its application to many other substrates: cartons, plastic,...
BMS Spring Release
We are only a few weeks away from releasing the Version 2.0 of the Brand Monitoring System ( BMS), our online platform which allows brands to control and monitor brand protection activities. AlpVision BMS was first released in 2017 with...
IACC Spring Conference

Boston, Massachusetts, will be hosting the IACC’s 2019 Annual Spring Conference! The year 2019 is very special for the IACC as it is the organization’s 40th anniversary! 40 years is a very impressive achievement for an organization in the anticounterfeiting...
Full Android Compatibility

Historically, the AlpVision authentication applications for Cryptoglyph and Fingerprint were released for the iPhone. We developed our first Android version back in 2014. Since then, we have seen a growing interest for the Android version.
Artificial Intelligence at AlpVision
Artificial Intelligence (AI) is everywhere, including at AlpVision. In the last few years, we have developed several machine learning approaches for our authentication software. For specific problems, AI substantially increases the performance of our apps.
AlpVision Joins IACC

We are pleased to announce that starting in January 2019 AlpVision is an active member of the International AntiCounterfeiting Coalition (IACC), a Washington, D.C.-based nonprofit organization devoted solely to combating product counterfeiting and piracy.
New Offices in Shanghai

Since the opening of our office in Shanghai, AlpVision has recorded excellent sales results in China. By the end of December, we will relocate to the Swiss Center in Hongquio which features ultra-modern offices in the heart of Shanghai.
New Website Live for Cryptoglyph
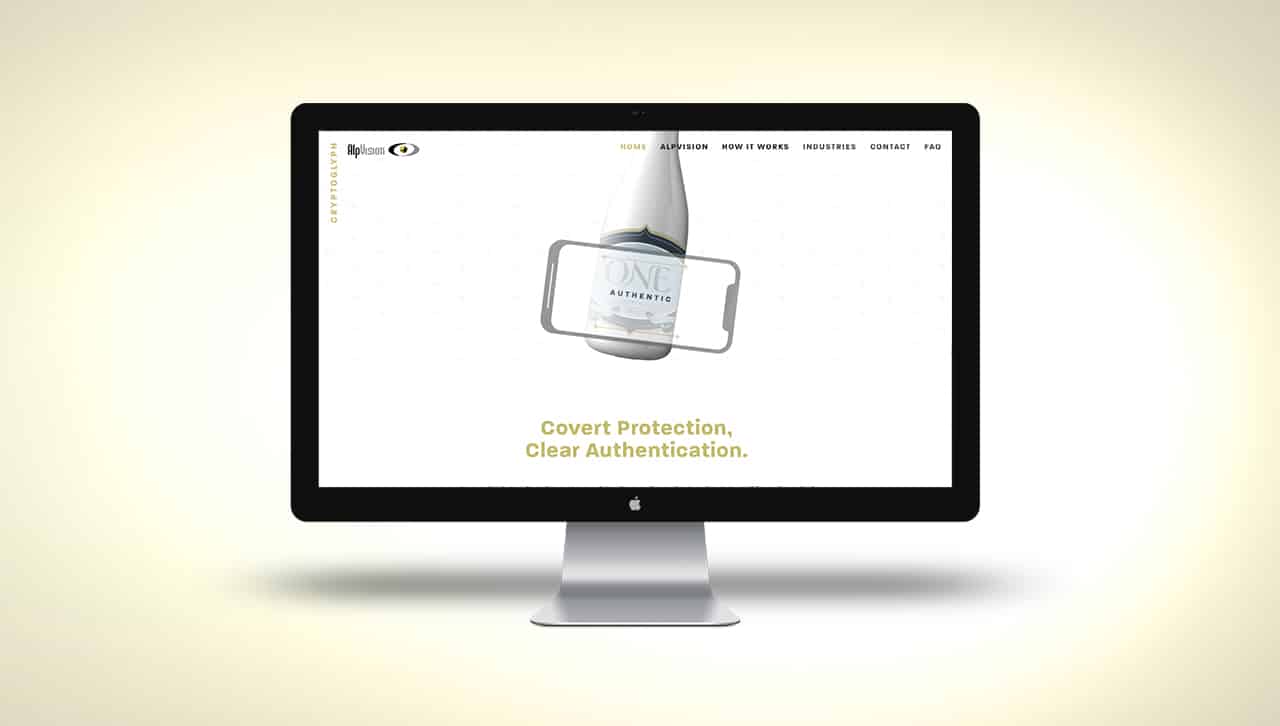
It is with great pleasure that we invite you to visit our new website dedicated to Cryptoglyph, our flagship anticounterfeit technology for the protection of printed products and documents. Cryptoglyph’s story started in 2001 when Dr. Fred Jordan and Dr....
Join us in Frankfurt!

On the 4th and 5th of December 2018, AlpVision will participate in the 2nd Brand Protection Congress in Frankfurt, Germany. 120 attendees are expected with representatives of more than 25 countries.
CBC Anti-Counterfeit Committee

A new user group bringing together over 10,000 members of the Chinese cosmetic industry has been created. This group named “CBC Anti-Counterfeit Committee”will run under the presidency of Lucien Zhao from AlpVision China.
Don’t miss AlpVision at IACC and GBPS

With events in the US and in Europe, October is going to be a busy month for AlpVision. The first event is GBPS - 9th Annual Global Brand Protection Summit - in Amsterdam on October 10th and 11th, 2018.
Two new patents have been granted to AlpVision!

The first one is titled “Method intended for preventing forgery or alteration of a printed or engraved surface”. This patent, granted on July 30th, covers European countries and reinforces the protection coverage of our Cryptoglyph technology, which enables printing of...
Iphone authentication on solid color

We have just completed the successful deployment of a new security feature inspired by both the Cryptoglyph and the Secured QR-Code technology.
AlpVision SA is ISO 9001:2015 certified

It is our great pleasure to announce that as of July 3rd, 2018, AlpVision SA is ISO 9001:2015 certified. The certification was performed by SGS (Société Générale de Surveillance SA), known as the global leader in certification. ISO 9001 is...
AlpVision is ISO 9001:2015 Certified

AlpVision is proud to announce the granting of a new patent. This US patent 10,019,627 B2 was granted on July 18, 2018 and describes a new digital authentication technology based on the analysis of two images.
Authentication Solutions for Bottles

In the past few years, AlpVision has developed an entire family of solutions to authenticate bottles. In particular for automotive lubricants and the distilled spirits industry, we adapted the Cryptoglyph technology so that it would work successfully with labels, plastic...
Fingerprint Technology Improvement

One of our flagship authentication solutions is the AlpVision Fingerprint. It enables consumers to instantly authenticate a product just using the micro-imperfections made during its manufacturing. Only a smartphone is needed, without any additional devices like lights or lenses.
Artwork Fingerprint, a new authentication technology

AlpVision has been securing billions of products for many years using invisible features. Those features are either created on the packaging (Cryptoglyph) or are already existing on the product (Fingerprint). There is a third class of feature, the most obvious...
AlpVision Compatible with WCO IPM Version 2.0

Some time ago the world customs organization released API Version 2.0 of IPM, their web and mobile platform. This platform allows rights holders to share relevant product information and facilitates online verification of product authenticity.
Meet us in London

Do not miss an opportunity to meet with AlpVision this spring and discover our brand-new solution to protect QR Codes and bar codes.
Innovation – shrink-sleeve authentication

We are happy to announce that we have successfully completed the qualification of a new type of packaging that can now be protected with our technologies. Indeed, we adapted our solution to the so-called “shrink-sleeve”, which are flexible plastic films...
Secured QR-Code to Combat Scams and Counterfeit Products

AlpVision Secured QR code add anti-counterfeiting functionalities to products featuring a QR code. This new feature therefore simultaneously provides track and trace and anti-counterfeiting.
New! Fake & Genuine Detection

All the AlpVision authentication applications automatically detect authentic products by analyzing in real-time the video stream coming from the camera of the smartphone, giving the user instant authentication of the product.
Patent Granted for Unique AlpVision Technology

AlpVision had a new patent granted in the US for its Fingerprint technology. Entitled “Mean for using microstructure of materials surface as a unique identifier”, this patent covers the latest Fingerprint technology advances while building upon the unique imaging and...
Novel QR Code for Authentication

At the Brand Protection Congress on Nov. 22, 2017, AlpVision announced the release of a QR Code that can be used for authentication. This solution instantly detects when a QR Code has been copied.
Anti-Counterfeiting Reports
On 15th of November, QyResearchReport published a detailed report addressing anti-counterfeit packaging solutions for electronics. It examined top players supplying anti-counterfeit solutions, including AlpVision’s Cryptoglyph.
iPhone 8 Full Compatibility
Historically, AlpVision has always supported the Apple iPhone series since the iPhone 4. Therefore, all our applications are carefully tested on each hardware model version as well as each main iOS release.
Meet AlpVision in November
This coming November will be a busy month. 30 days and 3 opportunities to meet AlpVision and experience our solutions first hand. The first two opportunities take place in London, UK, and the third one in Munich, Germany. The first...
In the News
Our company has been active with authentication technologies for over 15 years, and billions of products are protected with our solutions each year, produced by over a hundred suppliers worldwide. As a result, there are numerous articles on the internet...
Should Product Authentication Be at the Consumer’s Fingertips?

According to a recent white paper by Authentix, an authentication and information services company, brand owners should not delegate nor should they rely on consumers to authenticate products. Authentix first argues that consumers do not want to scan QR codes....
Discover AlpVision’s Brand Monitoring System
Implementing an authentication solution is a necessary but insufficient step to effectively protect brands against counterfeiting. Once a solution has been put in place, it is extremely important to use it, collect its results and monitor its effectiveness. During the...
8th Global Brand Protection Summit
If you have not met with us for some time, the 8th Global Brand Protection Summit would be the perfect place. The talks take place in Amsterdam, The Netherlands, on 3rd and 4th October 2017, the Global Brand Protection Summit...
European Union Tobacco Packaging Directive
The 2nd Stakeholder Meeting on the Implementation of the European Union Tobacco Packaging Directive was held on the 15th of May 2017. The purpose of this directive is to define solutions on tobacco products in order to be able to...
Why mass visible serialization is failing

Mass visible serialization; i.e mass serialization with visible tags is not working for product authentication. Here we explain why.
Meet us at EPHJ/EPMT/SMT in Geneva
As every year around this time, we invite you to visit us at the EPHJ event, taking place in Geneva, Switzerland, June 20 to 23rd, 2017. EPHJ (Professional Watchmaking and Jewelry Environment) takes place together with the EPMT (Microtechnology Professional...
AlpVision Included in New Anti-counterfeiting Reports
MarketsandMarkets just published their global forecast for the worldwide anti-counterfeit market. This report predicts that the main market territories in 2021 are expected to be dominated by Asia, followed by Europe and Norh America.
Smartphone Offers Point-and-Authenticate Capability
Product authentication has been, and still is, logistically very challenging. For example, banknotes require both robust, yet easy-to-use anti-counterfeiting and security measures to promote counterfeit deterrence and consumer adoption. Together with a steady rise in the number of sophisticated counterfeiters,...
Meet us in Europe and China
Two continents, two opportunities to discover our product authentication services before the summer. The IACC 2017 Annual Spring Conference will be held in Barcelona, Spain, May 17-19 this year. The IACC conference combines the industry's most up-to-date information and best...
Two Opportunities to meet AlpVision
March 2017 will be a very busy month for AlpVision commercial team with two events in Germany. The first event will take place in Munich on March 16th and 17th. Called the Brand Protection Excellence Forum, this event is a business...
New US Fingerprint Patent Granted
AlpVision Fingerprint is being used worldwide to protect molded products against counterfeiting. For example, this technology protects such diverse items as flip-off caps of vials, precious metals, and bottle closures. Using intrinsic features of the product, AlpVision Fingerprint is one...
Serialization vs. Aggregation: A DSCSA Progress Update

In April 2016, we reported on Title II of the Drug Quality and Security Act (DQSA), known as the Drug Supply Chain Security Act or DSCSA. Enacted in 2013 by the U.S. Food and Drug Administration, the DSCSA requires a...
The Evolution of Authentication Technologies

At the beginning of the millennium, product authentication was mainly done with a special tool or with the naked eye. The former solution was reliable but cumbersome while the later became less and less useful as simple copies became very...
Blister Pack Authentication with mobile phones
Authenticating secondary packaging, such as folding boxes, is very often a first choice for a brand as one is not required to open the box to authenticate the product. However, in addition to the multi-layer paradigm, pharmaceutical companies have realized...
2016 A Look Back
As the year comes slowly to an end, it is time to have a brief look at an exciting period. With over 10 billion products protected per year with our security features, we are very proud to hold our position...
Cigarettes Top the List of Detained Articles in the EU in 2015

The European Commission recently published the 2015 Report on EU Customs Enforcement of Intellectual Property Rights.I While the number of cases (a ‘case’ refers to a detention that may contain one or more articles) dipped slightly in 2015 (95,194 in...
The Success of the Smart Embossing
The launch of our Smart Embossing technology last month has generated an impressive amount of interest among our customers and prospects. Initiatives such as the EU’s Falsified Medicines Directive and Tobacco Products Directive highlight the need for authentication and track...
Cryptoglyph on Seal Liner
The Cryptoglyph technology is an invisible marking that has been used for many years to authenticate boxes and labels.
AlpVision in China – The experience of a new market
One year ago, in October 2015, AlpVision set up its new offices in Shanghai with the intent to expand the brand protection business. Compared to our marketing experience in Western countries, we have been happily surprised by the immediate brand...
Counterfeit Medicines in Asia: Damage Suffered by Japanese Pharmaceutical Companies Abroad

Although counterfeit medicines affect every region of the world, many drug-counterfeiting rings originate in Southeast Asian countries, such as Cambodia, Thailand, Laos, Vietnam, and China.I Many factors tend to bolster this situation, including weak enforcement, lack of education and training, poverty,...
Smart Embossing – New Overt Security Technology
Smart Embossing is a totally new security technology dedicated to product authentication by consumers. This new solution builds on the key features of our current offerings: user friendliness, security, simple deployment and affordability.
Meet AlpVision in Europe/USA/Asia
Don’t miss the three upcoming opportunities to meet with AlpVision and discover our new security feature Smart Embossing. The first occasion is at the IACC's 2016 Annual Fall Conference which will be held in Scottsdale, AZ, USA, October 19 to...
New patent granted in Hong Kong
AlpVision SA has obtained a new patent protecting the Fingerprint technology in Hong Kong. The patent, ID 09102325.3, is accessible online at the Hong Kong patent office using publication number 1122887. This patent is based on the earlier European filing...
AlpVision celebrates its 15th year anniversary
AlpVision is proudly celebrating its 15th anniversary in June 2016. When we founded AlpVision in 2001, the company was a very early-stage startup in a garage. We had theoretical background related to steganography based on the Ph.D. work of Dr....
Global Brand Protection Summit in Amsterdam
AlpVision will again exhibit at the coming Global Brand Protection Summit event in September 21-22 to be held in Amsterdam. This event specifically addresses issues related to brand protection across a wide range of industries including food, beverages, automotive, cigarettes,
Mapping Out the Tobacco Products Directive
Dated June 6, 2016, a “Roadmap” was published on June 9th on the EU Transparency Register covering implementing measures and delegated acts under Articles 15(11), 15(12) and 16(2) of the Tobacco Products Directive (TPD) 2014/40/EU.I In E.U. terms, “roadmaps” are...
President Schneider-Ammann Visits AlpVision China
President Johann Schneider-Ammann, along with a high-ranking delegation of 60 people, recently participated in the ribbon-cutting ceremony of the newly created Machinery, Trade and Business Center, a member of the Swiss Center Shanghai (SCS) family located in
AlpVision’s Counterfeit Protection on Tin Capsules
In 2015, AlpVision released a new solution to protect metallic closures. In this time of counterfeit wine bottles, AlpVision adapted its Cryptoglyph technology to offer counterfeit protection on tin capsules. Tin capsules are typically found on a small, but top-notch...
AlpVision Expands Activities in Japan with ISID
AlpVision is proud to announce the signature of a distribution contract for its authentication technology with the Japanese company ISID.
What Do Pharma Safety Regulations Look Like in the U.S.?

On February 9, 2016, the countdown to implementing the safety measures mandated by the Falsified Medicines Directive (FMD) Delegated Act started. This Act, which will come into force in early 2019, imposes item-level serialization and tamper evidence on pharmaceutical products...
AlpVision Fingerprint: Wear and Time Sustainability
Fingerprint, developed by AlpVision, leverages the naturally occurring surface irregularities found in a mold and uses these unique characteristics to authenticate a variety of molded materials, including plastic and metal. The solution has been deployed successfully at industrial
FMD Delegated Regulation (EU) 2016/161 Published

The publication of the Delegated Regulation (EU) 2016/161I supplementing the Falsified Medicines Directive (FMD) 2001/83/EC and its amendment 2011/62/EU was finally published in the Official Journal of the European Union on February 9, 2016. This date marks the three-year window in...
Let’s Meet at Anti-counterfeiting Technology Forum & Exhibition!
Join us this spring for the New International Anti-counterfeiting Technology Forum & Exhibition on April 21-23 in Shanghai, China. On May 18-20, head over to Orlando,
Package Wrapping Is No Barrier to Cryptoglyph Detection
Today, many products are wrapped in protective plastic, such as see-through shrink-wrap and protective polypropylene film, to safeguard and cushion them. Without taking the contents of the package out, authentication of wrapped products becomes a real challenge.
AlpVision Granted New European Patent for Fingerprint
On November 4, 2015, AlpVision was granted a new European patent EP2024899B1 protecting the core innovation of our Fingerprint technology. This patent protects the key characteristic of AlpVision’s Fingerprint technology, which is leveraging the microstructure that naturally
Financial Impact of the FMD Delegated Act

Our previous blog article (Cost-Benefit Analysis of a ‘European Hub’ for Medicine Authentication) analyzed the cost-benefit of creating a European hub – the European Medicines Verification Organization or EMVO – to link national verification systems throughout Europe and facilitate the creation...
AlpVision Gains Clout in 2015 ACF Reports
Over the past year, AlpVision was often published in some of the leading reports addressing the anti-counterfeiting (ACF) industry. Written by experts, these independent and insightful reports review current technologies and solution providers, and make market forecasts.
AlpVision Launches New Smartphone Server Solution
When AlpVision was first founded, our solutions were deployed on PC and product authentication was performed using document scanners. Today, our product authentication systems run on smartphones, using the device's camera, flash and onboard processing power.
Cost-Benefit Analysis of a ‘European Hub’ for Medicine Authentication

As described in our previous blog (New Developments in the Fight Against Falsified Medicines), the European Medicines Verification Organisation or EMVO was founded in February 2015 as a means to help EU Member States implement the requirements set out by the...
European Commission Innovation Report
The Business Innovation Observatory of the European Commission released this year a study on Traceability Across the Value Chain:
AlpVision Opens New Office in China
When it comes to product counterfeiting, China is considered as one of the world’s hotspots. According to the WTO, 2% of all world trade accounts for counterfeit goods, and this number is steadily increasing. As the world's leader in digital...
New Developments in the Fight Against Falsified Medicines

In addition to the recent publication of a draft delegated act laying down the technical specifications of the safety features, two other major developments have taken place since the EU Falsified Medicines Directive (2011/62/EU) came into effect in January 2013....
Draft EU Falsified Medicines Directive Delegated Act Published

The Falsified Medicines Directive (2011/62/EU) adopted in June 2011 and put into force in January 2013, calls on the European Commission to prepare and adopt delegated acts that will lay down the technical specifications of the safety featuresI, determine the...
New US Patent Granted
AlpVision is proud to announce that a new US patent was granted, entitled “Means for using microstructure of materials surface as a unique identifierâ€. The new patent US 8180174 B2 protects the core processes that make up AlpVision’s Fingerprint solution....
AlpVision Fingerprint Ventures Into New Territory
AlpVision Fingerprint has experienced more revenue growth than any of our technologies over the past few years. The reason behind this trend lies in its simplicity. Instead of applying a marking, this authentication method relies on the imperfections that naturally...
Covert Security Features in EU Tobacco Control – comparison

On May 7, 2015, the European Commission published an analysis and feasibility study on the existing traceability and authentication solutions applicable to the tobacco industry. The report was successful in parsing the “marketing fluff” from the “real stuff” and emphasizing...
Meet AlpVision in Amsterdam and Denver, CO
Learn first-hand about AlpVision's instant product authentication solutions at two upcoming events this fall! AlpVision will be participating in the 6th Annual Global Brand Protection Summit (GBPS) on September 16-17, 2015, in Amsterdam. GBPS runs under the motto "Developing a...
Feasibility Report on EU’s Traceability and Authentication of Tobacco Products: A Look at Covert Security Features
In response to Articles 15 (Traceability) and 16 (Security feature) of the second Tobacco Products Directive 2014/40/EU, the European Commission published on May 7, 2015, an analysis and feasibility report defining the technical standards for traceability and authentication of tobacco...
Cryptoglyph on Metallic Closure is now available
The vast majority of our customers print AlpVision's Cryptoglyph technology on paper or carton. And so far, our solution has been remarkably successful protecting packaging and labeling. In recent months, we have made great strides in testing and
AlpVision Exhibits in Shanghai
From April 23-25, 2015, the International New Anti-Counterfeiting Technology Exhibition took place in Shanghai, China. The event was held together with the CSITF-China Shanghai International Technology Fair, a gathering of over 900 exhibitors and 38’000 visitors.
iPhone QC Tool Update
Although Cryptoglyph is invisible to the naked eye, AlpVision’s authentication feature can be instantly detected with a smartphone and a proprietary application.
AlpVision: Looking Back, Looking Ahead
2014 was an extremely interesting year for AlpVision, not only from a technological perspective, but also from a financial one.
Cryptoglyph & Innovation
AlpVision is proud to announce the first roll out of a Cryptoglyph-based product authentication and printing quality control system using the iPhone.
Embedded QA Software Update Released
AlpVision’s Embedded QA (EQA) is a printing quality measurement device used by hundreds of Cryptoglyph-qualified printers worldwide. Portable, lightweight and user-friendly,
AlpVision Apps compatible with iPhone 6 and iPhone 6 Plus
On September 9, 2014, Apple released the iPhone 6 and iPhone 6 Plus. According to Apple™ press release, the two new devices are the biggest advancements in iPhone history, featuring two new models with stunning 4.7-inch and 5.5-inch Retina HD...
Three new AlpVision partners serving Asia
AlpVision is proud to announce three new partnerships to help commercialize its anti-counterfeiting and product authentication solutions in the Asian markets. AlpVision will be working with swiss bridge consulting (Switzerland, Hong Kong, Vietnam), an independent company with a
Two market studies list AlpVision among leading anti-counterfeiting companies
At the beginning of 2014, visiongain published a new report on “Anti-counterfeit packaging technologies market forecast 2014-2024â€. The report identifies trends and segments with high revenue potential, analyzes submarkets, and reviews solution and technology providers. AlpVision is extremely pleased to...
AlpVision solutions now run on Android Smartphones
AlpVision provides two covert authentication solutions. Although invisible to the naked eye, both solutions can be detected instantly using an iPhone running on our dedicated application. In 2001, we started offering product authentication using document scanners and PC software.
Meet us at AAPEX 2014, Las Vegas, November 3-6
AAPEX – Automotive Aftermarket Products Expo – is one of the largest automotive aftermarket products expos in the US. During the three days
New AlpVision Anti-Counterfeiting Blog
We are pleased to announce the launch of our new Anti-Counterfeiting Blog, a space where AlpVision co-founders Fred Jordan and Martin Kutter will cover and share opinions about anti-counterfeiting and brand protection news. The blog, which can be found here,...
Multiple printers successfully complete Cryptoglyph printing qualification program
AlpVision is glad to welcome three more folding box and label suppliers among its qualified printers. Protecting printed packaging components with AlpVision Cryptoglyph technology is very easy and does not change the printing process.
iPhone based quality control tool
AlpVision has released the first version of a new system for quality control for the Cryptoglyph printed in varnish. The new tool enables a real-time and user-friendly assessment of the printing quality of AlpVision’s invisible marking flagship. The marking is...
Meet us at EastPack, New York, June 10-12, 2014
EastPack, the East Coast Packaging Industry Event, returns to New York on June 10-12, 2014. Don’t miss this unique opportunity to meet with Quincy Mattingly, regional manager, to get a first-hand demonstration of instant product authentication using an iPhone.
AlpVision hands-on lab at the Petroleum Packaging Council
AlpVision is glad to announce the first official authentication hands-on lab at one of our qualified printer partners exhibit during the Petroleum Packaging Council (PPC) Spring Meeting 2014 & Tradeshow, March 17th, in Tampa, Florida.
AlpVision at IP Protect Expo 2014, March 11-12 in London
AlpVision is set to participate for a third year in IP Protect Expo 2014 (www.ip-protectexpo.com), an annual networking space for the worldwide intellectual property and brand protection community, scheduled to take place March 11-12, 2014 at the Business Design Centre...
AlpVision Fingerprint compatible with iPhone macro lenses
The Fingerprint AlpVision technology enables to authenticate molded or die stamped parts using just an iPhone. This authentication solution covers for instance plastic parts obtained by injection molding like caps, circuit breakers, flip-off caps,
AlpVision Fingerprint characterized by Charmille Surface
AlpVision Fingerprint technology authenticates molded or stamped parts by automatically recognizing naturally occurring microscopic features found in a mold. Interesting and convenient, this solution allows brand owners and experts in the field to identify genuine from counterfeit products using an...
iPod Authentication? Yes, it Works!
Just a few months after the release of the first iPhone, Apple launched the iPod touch. Essentially, an iPod touch is an iPhone without phone capabilities that targets the gaming and music user segments. As AlpVision’s Cryptoglyph and Fingerprint technologies...
Mobile Solution deployed on US Pharmaceutical Products
AlpVision has just successfully completed implementing its Cryptoglyph authentication mobile solution for a large US based pharmaceutical company. Printing the Cryptoglyph did not alter in any way the printing process, which was printed in classic flexography using standard overprint varnish....
AlpVision to Exhibit at 2013 IACC Annual Fall Conference
Based on the success of the Spring conference, AlpVision will exhibit again at the International AntiCounterfeiting Coalition (IACC) 2013 Fall Annual Conference to be held on October 16-18 at the Westin Kierland Resort & Spa in Scottsdale, Arizona, USA. The...
MobileIron: A Secure Alternative to Deploying AlpVision™s Mobile Authentication Application
Because AlpVision™s brand protection and product authentication solutions are covert, AlpVision does not make its mobile authentication application available on the App Store. In addition, because each application is customized to meet brand owners™ unique authentication needs, customers prefer to...
AlpVision Mobile Application Now Authenticates Footwear
According to the National Chamber Foundation, the estimated annual industry loss due to footwear counterfeiting in the U.S. is $12 billion. To combat counterfeit shoes, AlpVision offers the footwear industry its patented Fingerprint technology, a breakthrough covert anti-counterfeit solution that...
AlpVision Featured in Labels & Labeling: Smart Technology
AlpVision is featured in the July issue of Labels & Labeling in an article entitled, Smart Fight Against Counterfeits by Carol Houghton. The piece discusses improvements made to AlpVision's iPhone application now capable
PPLC Spring 2013 Meeting at Amgen in Thousand Oaks, CA
AlpVision was recently invited to speak at the IoPP (Institute of Packaging Professionals) PPLC spring 2013 - Pharmaceutical Packaging & Labeling Committee - meeting at Amgen in Thousand Oaks, CA. Under the theme of Serialization & Anti-Counterfeiting, Quincy Mattingly, Regional...
AlpVision Marks World Anti-Counterfeiting Day 2013
Established in 1988 by the Global Anti-Counterfeiting Group (GACG), World Anti-Counterfeiting Day gives us a chance to show you how you can help combat counterfeiting. Tips for Brand Owners AlpVision's product authentication and counterfeit protection solutions are developed with your...
The WCO announces that AlpVision is now IPM Connected
The World Customs Organization (WCO) was host to the 7th Global Congress on Combating Counterfeiting and Piracy, held in Istanbul, Turkey, from April 24-26. On this occasion, the WCO introduced the new mobile version of the Interface Public-Members (IPM),
AlpVision’s Mobile Authentication Solution Improved to Detect Standard Cryptoglyph-secured (dots) Packaging and Labeling
Offset printers, rejoice! Until now, AlpVision's iPhone authentication application detected varnish Cryptoglyph, a pseudo-random pattern of invisible micro-holes (40-80 microns)
Instant Brand Authentication at IP Protect Expo 2013
AlpVision participated for a second year in IP Protect Expo on February 5-6, 2013 in London, UK. Exhibiting in Stand #11, AlpVision showcased its iPhone authentication application,
AlpVision iPhone Authentication App Improved to Authenticate Vial Labels and Other Curved Surfaces
AlpVision has given its iPhone authentication app a welcomed update,
AlpVision in Anti-Counterfeiting Top 25
AlpVision is among Global Identification's Top 25 Suppliers of anti-counterfeiting & product security technologies!
New! AlpVision’s Authentication App Now Available on iPhone 5
A year ago, AlpVision released an iPhone 4/4S application capable of authenticating AlpVision's digital invisible anti-counterfeit technologies. AlpVision's mobile authentication solution applies to a wide range of items, including packaging, labeling and plastic molded parts. Today, AlpVision has successfully ported...
Janoschka Innovation Day for Brand Security Solutions
Dr. Fred Jordan, Co-founder and CEO of AlpVision, was invited to speak at Janoschka's Innovation Day for Brand Security Solutions on November 30, 2012, in Kippenheim, Germany. Janoschka is a global leader in prepress solutions.
AlpVision iPhone App Now Authenticates Blister Packs
In response to industry needs, AlpVision has adapted its iPhone authentication application to blister packs with cavities as small as 5 mm in diameter and covered with dense artwork. Combined with a readily available and inexpensive macro lens
AlpVision at Imaging Supplies Coalition Conference
AlpVision participated in the Imaging Supplies Coalition (ISC) 14th International Anti-Counterfeiting Conference held on August 19 through 21 in Las Vegas, NV. The conference was attended by approximately 75 industry specialists and coalition members, including Brother, Canon, Dell, Epson, HP,...
AlpVision Releases ScanHelper, No Installation Required.
AlpVision, a world leader in digital solutions for product authentication, is releasing ScanHelper, a standalone Microsoft Windows computer program that allows brand owners to easily scan AlpVision-secured packaging and products using a
AlpVision Granted U.S. Patent for Means for Using Microstructure of Materials Surface as a Unique Identifier.”
The United States Patent and Trademark Office granted U.S. Patent No. 8,180,174, covering AlpVision's Fingerprint technology or the use of material or document microstructures for tracking, tampering and
AlpVision Marks World Anti-Counterfeiting Day 2012
Established in 1988 by the Global Anti-Counterfeiting Group (GACG), World Anti-Counterfeiting Day is an opportunity to raise awareness of the international costs of counterfeiting and piracy. On this day, AlpVision would like to call attention to the economic, social and,...
AlpVision Founders Published in Esteemed Scientific Publications.
Dr. Martin Kutter, President, and Dr. Fred Jordan, CEO, were recently published in two esteemed scientific publications. The first article titled Industry-suitable Technologies to Protect Pharma Products against Counterfeiting appeared in the March 2012 edition of the peer-reviewed International Pharmaceutical...
AlpVision and Nolato Medical Announce Joint Collaboration
Coined the crime of the 21st century counterfeiting of pharmaceutical products accounts for an estimated $600 billion in global trade. Among other preventive measures, packaging can play an important role in deterring copying, tampering or package pilferage. In response, AlpVision,...
Pro Carton Congress and Anti-Counterfeit Conference Wärzburg
Dr. Fred Jordan, Co-founder and CEO of AlpVision, recently presented Digital Solutions for Packaging Authentication at the International Pro Carton Congress in Duesseldorf, Germany, and Authenticating Medicines with an iPhone 4 App at the 3rd University of Waerzburg Anti-Counterfeit Conference...
Exhibition at Interphex 2012 in New York, USA.
AlpVision completed a successful three-day exhibition at Interphex 2012, from May 1-3, 2012, at the Javits Center in New York, NY. Interphex is the leading trade event to create innovative solutions that improve manufacturing
Instant detection of Varnish Cryptoglyph with iPhone
AlpVision announces the launch of an iPhone 4 instant product authentication application capable of detecting its Varnish Cryptoglyph solution, an invisible marking
IP Protect Expo 2012 in London, UK.
AlpVision completed a successful two-day exhibition at IP Protect Expo, from March 28-29, 2012, at the Business Design Centre in London, UK. IP Protect Expo is the definitive intellectual property and brand protection trade exhibition by industry, for industry. AlpVision...
New premiere at AlpVision: an iPhone4 application for identification of counterfeited molded parts on-the-fly.
AlpVision has made a big step forward in providing mobility for on-the-fly Genuine or Fake verification of molded parts. This applies to many items, for example for Fast Moving Consumer Goods containers, which are present in millions of stores worldwide....
AlpVision, top finalist at the Swiss Technology Award 2011
According to INSEAD 2011 GII (Global Innovation Index), Switzerland is the most innovative country in the world. AlpVision is therefore especially proud to have been selected as a top 3 finalist from 70 innovative projects for the
GSS – Global Secure Summit in Prague, Czech Republic.
Brand security is a worldwide problem that is increasing and affecting many successful companies across all industries. Today, more than ever, companies need to combat security risks before it is too late.
Embedded QA”: a new Quality Assessment device for Cryptoglyph printers.”
This new device developed by AlpVision will provide packaging printers and converters with a portable Quality Control device with the goal of verifying the presence of the Cryptoglyph® invisible marking. The (rechargeable) battery powered system enables testing of offset plates...
Handbook of nutraceuticals Volume II – chap. 21 by AlpVision
Edited by Yashwant Pathak Ph.D., Chair and Professor at Sullivan University College of Pharmacy, at the CRC Press, ISBN 9781439823682, Handbook of nutraceuticals Volume II (newly edited on May 16, 2011) describes various processes used in nutraceutical manufacturing. As for...
Big success for AlpVision at Interphex 2011 in New York, March 29-31.
Building on its North American presence through the recent opening of offices in the Willis Tower (formerly Sears Tower) in Chicago, AlpVision presented at Interphex 2011 its full range of digital on-packaging and on-dosage product authentication solutions.
Two more pharma manufacturers rely on AlpVision anti-counterfeiting solutions.
Two other main pharmaceutical producers of branded medicines have signed licenses for roll-out of AlpVision's covert digital solutions for product authentication. The worldwide deployment will take place in the course of 2011.
Debiopharm relies on AlpVision anti-counterfeiting technology
Debiopharm Group, a leader in the field of drug development and of innovative drug delivery processes, has decided to use an AlpVision digital solution for product authentication. This strategic decision will involve the setup and implementation of a tool to...





