Pharmaceutical
counterfeiting
what are
the actual risks
Globalized pharmaceutical logistics networks are threatened by the scourge of counterfeits, especially after the COVID-19 pandemic. The remedy is efficient, proven and easy to deploy anti-counterfeiting solutions.
The PHARMACEUTICAL MARKET AND SUPPLY CHAIN CHALLENGES
CURRENT SCENARIO AND NEW TRENDS
Around 10% of pharmaceutical drugs are counterfeit, at a cost of up to $200 billion annually. Interpol calculates that this volume of counterfeit drugs has a death toll of 1 million people every year (WHO, 2018).
The immediate decline in revenue the happens after the patent on an innovative drug expires, the so-called patent cliff, is a major issue facing the pharma industry, especially producers of blockbuster drugs. When the patent protection ends, generic manufacturers enter the market with drugs that are equivalent to the innovator’s drug, at a significantly lower price.
The average life expectancy is increasing in OECD countries, and the most interesting new trend in pharmaceuticals is that of Precision and Personalized Medicine (e.g., DNA sequencing of genes), which is expected to increase greatly in the coming years, especially in the realm of preventive medicine (Deloitte LLP, 2017).
DIFFERENT TYPES OF PHARMACEUTICAL FORMULATIONS
Pharmaceuticals can be categorized by traditional dosage/delivery forms (oral, ophthalmic, etc.) or by physical dosage form (solid, semi-solid, etc.). Finished products, such as prescription drugs, over-the-counter medicines and generics, contain compounds produced by chemical synthesis or plant-based compounds. Biopharmaceuticals, which are biologically based and harness cellular and biomolecular processes, represent the new frontier and include:
- Vaccines
- Blood, blood components and plasma derivatives
- Recombinant therapeutic proteins (including monoclonal antibodies)
- Genomics-based precision medicine
- Biomarkers (for reducing treatment overheads)
- Nanotechnology-based drugs and carriers
The complexity and diversity of treatments make it increasingly important to optimize and globalize pharma’s logistical network.
PHARMACEUTICAL SUPPLY CHAINS
A supply chain is the means by which a company ushers its goods from development to the marketplace to sell them and generate a profit. In the case of a pharma company it embraces all the organizational, operational and value-adding activities involved in manufacturing medications and getting them to the customer covering everything from new development to delivery to the hospital, retail pharmacy, or patient.
The biopharmaceutical industry’s logistical network is uniquely complex, involving matters such as:
- cold chain transports
- cell culturing
- cryopreservation
- intensive Quality Control (estimated 70% of elapsed time) (Deloitte LLC, 2020)
Track & Trace/Serialization
One feature of the pharmaceutical logistics network is serialization and the track-and-trace concept. Over the past 20 years, the industry has worked in many markets to secure its network by developing and implementing serialization standards, under which medications are given unique identifiers to secure the legitimate logistical network from infiltration by counterfeit, diverted, and adulterated medications. In serialization, a unique serial number is assigned to each saleable unit that can identify its origin, batch number, and expiration date. Markets have enacted regulations to enforce the use of unique identifiers, whether applying serial numbers at certain packaging levels or reporting batch data to national agencies. Serialization is mandatory in many countries (Europe, USA) but totally absent in others, including many in Africa.
AGGREGATION
Aggregation adds codes to the outside of cases and pallets to identify the individual pack–level medications inside. In other words, aggregation builds parent-child relationships from pallet to case to individual pack–level medications.
Goods serialization makes it possible to follow a product’s journey through the logistics network, enabling tracking and tracing and producing a record of its end-to-end lifecycle (and, indirectly, all its components, raw materials, etc.).
Serialization was introduced by governments (as described below in “Legislative Requirements”) to combat fakes and secure the logistical network, but for disparate reasons it has proved insufficient:
- Consumers deliberately sidestep the legitimate logistical network as reflected in the substantial growth of online pharmacies.
- Serialized barcodes are easy to copy.
- Many countries are not subject to serialization requirements. In fact, the highly regulated countries see only a minor impact of fakes.
- A welter of markets, formats, regulations, and guidelines has made serialization more an exercise in compliance than a real, efficient anti-counterfeiting strategy.
- Serialization systems are not integrated.

The risks of globalization and digitalization
Explosive growth in pharma spending has paralleled the globalization of the drug logistical network, which has introduced new players in diverse international markets, including contract-based suppliers, manufacturers, suppliers of raw materials, and trading partners, resulting in a “diversification” of logistics. Medicines change hands repeatedly and are subject to multiple transactions between production and the patient, with each transaction increasing the risk of sham, substandard goods infiltrating the supply chain. Some medicines enter the largely unregulated “gray market,” populated by secondary wholesalers, traders, and resellers, where the possibility greatly increases of sourcing improperly stored, diverted, contaminated, counterfeit, or falsified medicines.
The globalization of consumer markets, coupled with the rise of e-commerce platforms, has also opened new channels for fake medicines, including purchase and delivery via online “pharmacies” that sell prescription drugs directly to the consumer. The globalization of e-commerce has given rise to a digital gray market completely separate from the legitimate pharma logistical network.
Key players
Supply chains are more and more globally integrated and complex, involving (even if we exclude the early phases of Discovery, Research and Development) a huge number of players:
- suppliers
- manufacturers and contract manufacturers
- regulators (FDA, MHRA, etc.)
- pharmacy benefit managers (PBMs)
- healthcare group purchasing organization (GPOs)
- health insurers
- wholesaler distributors
- integrated Delivery Networks
- hospitals
- retailers/pharmacies
- patients
As a side note, the COVID-19 pandemic represents a black swan event that may force some of the above actors to rethink and transform their logistical network model. The world was taken completely by surprise, and, while the situation is still unfolding, a number of takeaways have already emerged.

Post–Covid 19 lessons learned
- Logistics networks rely excessively on a few sources of distribution, leading to drug shortages; Western countries still lead drug discovery and development but are no longer dominant in drug manufacturing, especially for active pharmaceutical ingredients (APIs).
- The security of logistics has become a matter of national concern; it is crucial to obtain more data on offshore API manufacturers (including volumes and quantities) (FDA, 2019).
- The diversion, theft, and counterfeiting of critical items, such as test kits, vaccines, and antivirals, have greatly increased and will continue to do so. (IDC, 2020).
- A resilient supply chain involves increased complexity, with regulations such as the Drug Supply Chain Security Act (DSCSA) adding red tape to how pharmas work with partners, distributors, and logistics providers. Resilience is required to meet track-and-trace, monitoring, and licensing requirements. Thus, big pharmas must fully digitize every part of their logistical network operations.
- End-to-end visibility across production and logistics is essential. This includes real-time production monitoring systems and advanced planning and scheduling tools in manufacturing as well as the ability to track and trace drugs—from materials to final item—as they pass through the business logistics pipeline.
- In terms of business agility, the goal is to evolve from reactive distribution to patient-centric demand. A logistical network that is purely reactive and reliant on inventory buffers performs poorly overall while visibility and agility allow pharmas to be proactively driven by demand and centered on patients. Wholesalers and third parties are the most concerned about the lack of business logistics management and drug provenance certainty, leading to counterfeit, diversion, and theft. (IDC, 2020).
The solutions to the above-listed supply chain challenges can be found among the new trends in how supply chains are orchestrated.
New trends
(Deloitte LLP, 2017, David Alvaro, 2020)
Most pharmas currently manufacture and distribute their own goods, but this reduces asset utilization rates and drives up logistics costs. Conversely, sharing manufacturing and logistics resources would be much more economical. One of the latest evolutions in business logistics management is the transformation of logistics from third-party logistics (3PL) to fourth-party logistics (4PL). The latter are strategic service providers (a.k.a. lead logistics partners, or LLPs) that manage warehousing, inventory, fulfillment, transportation, and logistics on behalf of their clients, often managing the entire logistical network from the production line to the point of final distribution.
Other recent initiatives include the sharing of supplier data among big players, such as GSK, Takeda, Teva, Boehringer Ingelheim, and Amgen, for sustainability purposes (although such information was once considered strategic and proprietary) (cf. the Responsible Health Initiative EcoVadis Team, 2020).
The optimization of logistics was one of the main drivers of last year’s megamergers and acquisitions, and blockchain technology offers promise for securing and increasing the overall visibility of the whole logistical network. There are many ongoing initiatives, including:
- the PharmaLedger project
- the MediLedger Network
For biopharma, the importance of logistics integrity goes beyond addressing counterfeit items, as key drug types need a chain of identity and a chain of custody, particularly in personalized medicines and gene therapies. To tackle this challenge, biopharma companies are investing in a combination of advanced innovations, such as blockchain and AI, to ensure the integrity of end-to-end business logistics management (Deloitte LLC, 2020; IDC, 2020).
THE SCOURGE OF COUNTERFEITS
Counterfeit pharmaceuticals are increasingly prevalent and profitable. Counterfeiters can produce spurious versions of all types of medicines, and these drugs may contain no active ingredient, harmful ingredients, the wrong drug, the wrong concentration, the wrong dose, or drugs past their expiry dates. All of these put patients at risk for treatment failure, harmful side effects, and dangerous drug interactions.
If the counterfeit pharma industry is worth as much as $200 billion annually, the illicit drug trade far exceeds that value. The successful marketing of counterfeits requires counterfeiters to penetrate logistics chains that, for the most part, are closely monitored by producers and regulators. While the wholesalers that are responsible for distributing most pharma medications are secure, thousands of second-tier distributors are more vulnerable to penetration by counterfeiters. Counterfeiters’ ability to package fake drugs in a way that mirrors genuine medications is crucial to their success as is their ability to make the items resemble the originals. Free trade zones have facilitated trade in counterfeit pharmaceuticals.
The challenges are particularly great in developing countries, where informal distribution is more widespread and less secure, but they have grown in almost all countries with the emergence of rogue online pharmacies. Unfortunately, despite the potential negative consequences, consumers have demonstrated a willingness to take risks by buying drugs online.
BRAND PROTECTION IN THE PHARMA CONTEXT
Protecting consumers from fake and potentially harmful drugs is the top priority; nonetheless, pharma brands also need to safeguard their reputations, revenues, integrity, and quality of goods. Brand protection
also embraces legal matters and elements related to intellectual property and trademarks. Pharma companies may be liable if consumers are harmed by counterfeit versions of their drugs.
Without an efficient brand protection strategy, consumers will not trust your drugs. Such a strategy includes but is not limited to robust safety solutions. It is also vital to:
- monitor the availability of relevant active pharma ingredients (APIs)
- surveille online distribution channels, particularly when linked to social media posts
- work with third-party organizations and law enforcement to assist with takedowns, evidence collection, and prosecution
- monitor the specific geographic areas where most unauthorized drugs have historically originated
- track potential gray market activity in any regions where a drug wins early approval

WHAT KIND OF COUNTERFEIT MEDICINES?
There is a great variety of counterfeiting approaches in the market for medicines. The most common are:
- counterfeit drugs
- counterfeit active pharma ingredients
- counterfeit packaging (primary, secondary, or tertiary)
- mimic drugs (unapproved drugs with trademark violation)
- undeclared API (a product where active APIs are present but undeclared)
When talking about fakes, it is important to employ common terms for classification (Nayyar, 2017), including:
- substandard
- falsified
- counterfeit
- diverted
- unregistered/unlicensed medical goods

SHORT- AND LONG-TERM NEGATIVE CONSEQUENCES OF COUNTERFEITING
Counterfeiting is sufficiently prevalent throughout history that it has been called “the world’s second-oldest profession.”
Counterfeit production has significant social and economic consequences. Most importantly, patients may not get safe or effective products and, consequently, may face significant risk. On the economic side, legitimate manufacturers of pharma goods suffer from patent and copyright infringement when counterfeiters, in reality, “hijack” the brand. Governments are affected through loss of tax revenue and the undermining of national healthcare systems. Considerable resources are required to combat the production of bogus medications. In addition, health insurers are defrauded and compromised.
Counterfeit medicines are unlicensed and thus, unlike genuine medicines, are not regulated or inspected. These drugs are often made in unhygienic conditions and without the rigorous testing and standards to which regulated medicines must adhere.
The World Health Organization has analyzed the socioeconomic, economic, and health impacts of substandard and falsified medical goods (WHO, 2018) as described below.
Socioeconomic impact
- costs in lost productivity to patients and households when they seek additional medical care
- lost income due to prolonged illness or death
- lack of social mobility and increased poverty
Economic impact
- economic losses for patients, families, health systems, and pharma companies
- waste of human effort and financial outlay across the health system
- increased out-of-pocket spending
Public health impact
- increased mortality and morbidity (adverse effects)
- progression of antimicrobial resistance
- higher disease prevalence
- loss of public confidence in medication and health systems

LEGISLATIVE REQUIREMENTS
There are two main legal frameworks for counterfeit protection, those of the European Union (EU) and US markets. We list the most important regulations below.
IN EUROPE
These two directives aim to prevent falsified medicines from entering the legal logistical network and reaching patients. They regulate the required safety features, the best practices of logistics distribution, the quality and traceability of imported APIs and excipients, and internet sales. As of 9 February 2019, marketing authorization holders in the EU are obliged to place two safety features on the packaging of most prescription medicines and some over-the-counter medicines: a unique identifier (a two-dimensional barcode) and an anti-tampering device.
IN THE USA
This act has similar requirements to those of the EU, with a deadline of 2023 established for having an electronic system to identify and trace certain prescription drugs as they are distributed in the US.

ANTI-COUNTERFEITING TECHNOLOGIES
Anticounterfeiting technologies may serve three distinct basic purposes:
anti-counterfeiting technologies can be characterized in the following ways:
- electronic
- printing
- marking/engraving
- technologies based on chemical-physical characteristics
- technologies based on mechanics
- ID coding and serialization
- distributed ledger
Some of the more interesting types of anti-counterfeit technology
Surface Fingerprint and Laser Surface Analysis
Invisible printing with standard inks
2D/3D holograms (diffractive optical variable image devices: DOVIDs)
Color-shift inks and, more generally, optical variable inks (OVIs)
Micro/nano-encrypted printing
Blockchain and crypto anchors
Optical authentication of physical unclonable function (PUF) tags
Unfortunately, it frequently rhymes with “cost.” High costs can derive from many factors: complexity, learning curve, novelty, exotic materials, etc.
Robustness and efficacy
It is commonly accepted that the robustness and efficacy of a counterfeit protection effort strongly depends on the variety and depth of the measures adopted. See for instance:
- The joint position of EFPIA, IFPMA, and PhRMA (2014):
No single solution will prevent the creation of fakes. Rather, a holistic approach consisting of covert and overt features; well-regulated, secure logistics; and appropriate laws and penalties to deter and punish counterfeiters is necessary to provide maximum patient protection. - The approach suggested in ISO 22383:2020: Guidelines for the selection and performance evaluation of authentication solutions for material goods (ISO, 2020):
An authentication solution can constitute a more robust solution when layered, and therefore it promotes the use of individual authentication elements in combination.

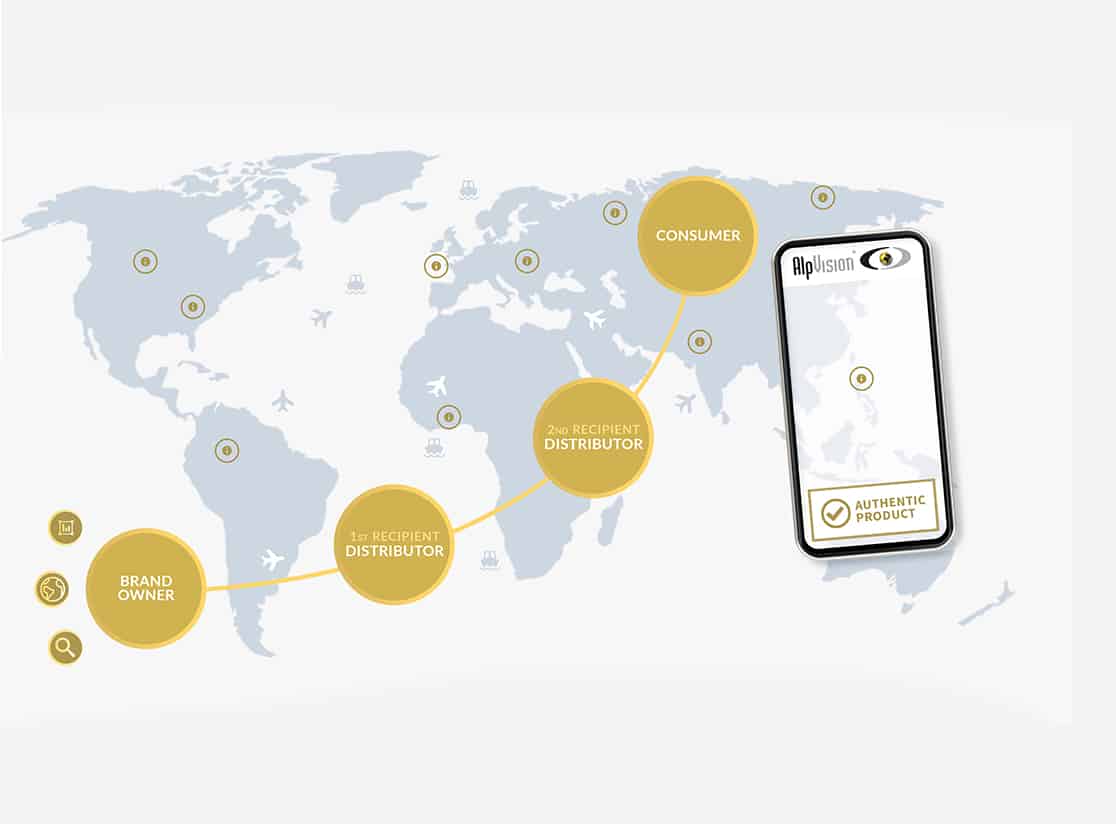
What does AlpVision offer ?
ALPVISION’S SOLUTION FOR PHARMA COUNTERFEITING AND BRAND PROTECTION
AlpVision, a leader in the brand protection market, provides invisible digital anti-counterfeiting and product authentication solutions to Fortune Global 500 companies across many industrial sectors worldwide.
Our offering
AlpVision offers a selection of covert anti-counterfeiting technologies suitable for print/packaging and physical goods:

CRYPTOGLYPH
Cryptoglyph is an invisible digital marking that is applied to printed products such as cartons, labels, and blister packs, using regular visible ink or varnish and standard printing processes (offset, rotogravure, flexo, laser, inkjet, etc.).
Simple and industry-proven, AlpVision Cryptoglyph protects billions of products across industries worldwide. It is commercialized under license agreements as an entirely customizable turnkey system.
Learn more about this solution here.
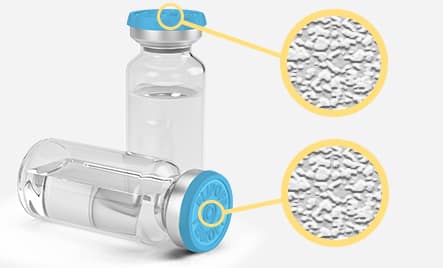
ALPVISION FINGERPRINT
With no changes to the standard production process, the AlpVision FingerprintTM technology leverages the intrinsic microscopic surface irregularities found on products and uses these unique characteristics as the means of authentication (no safety features are added). The anti-counterfeit solution based on this technology track and authenticates products. This solution applies to a wide variety of product parts, including molded bottles and caps, and in general to all kinds of packaging for pharmaceuticals.
Find out more about this solution here.
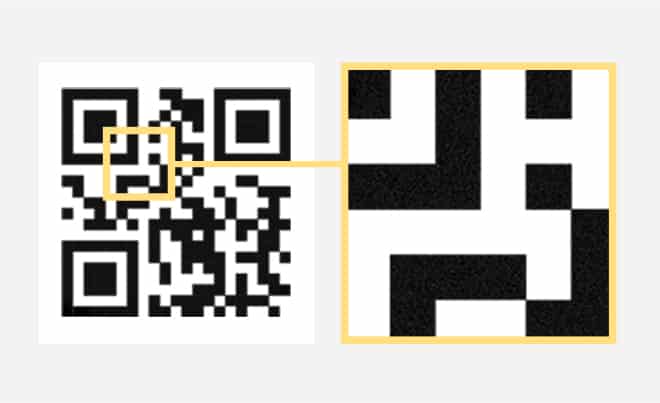
SECURED QR CODE
An invisible authentication layer developed for Track & Trace systems, it can be added to QR codes to enable authentication in the pharma supply chain. Such digital invisible marking can be applied to printed products, including cartons, labels, and blister pack.

BMS, Brand Monitoring System
AlpVision can integrate different security features and technologies into its anti-counterfeiting solutions, thus complying with the above-mentioned principles of robustness and efficacity. BMS is a server-based web application that allows brand owners to perform online monitoring and product authentication. BMS can manage AlpVision’s digital solutions for product authentication (Cryptoglyph, Fingerprint), as well as other third-party product authentication and brand protection solutions, such as QR codes, barcodes, two-dimensional matrix codes, and OCR.
All these technologies are natively AI fueled and can be further customized to meet the requirements of prospective life-science customers. Notably, regulators (such as the FDA) have started to approve AI software for use in healthcare.
KEY FACTORS OF ALPVISION’S COMPETITIVE ADVANTAGE
AlpVision’s commercial success stems on the following factors:
- Its security features are, by design, guaranteed to be highly resistant to copying.
- Authentication can be easily performed with a smartphone app(on both iOS and Android).
There is no need for exotic or expensive devices. - Our technology is easy, straightforward, and adds no burdensome manufacturing steps. The result is low implementation costs and low TCO.
- Security features are covert and completely invisible to the naked eye.
- Authentication results are immediate and of the highest reliability.
Specific to the pharmaceutical sector:
- AlpVision can work within the quality requirements of a pharma company’s critical suppliers and is backed by a Quality Management System that has been positively audited many times.
- AlpVision can provide GxP computerized systems that are compliant with FDA 21 CFR Part 11 regulations (and corresponding European regulations).
ALPVISION PROTECTS OVER 30 BILLION PRODUCTS EVERY YEAR
Since the early 2000s, AlpVision has a history of projects to protect pharmaceutical products from counterfeiting. Today, AlpVision protects over 30 billion products annually, include those of many Fortune Global 500 customers.

PATENTS HELD

PRINTERS WORLDWIDE

PRODUCTS PROTECTED

years of innovation
Counterfeit Products Market

PHARMACEUTICALS

TOBACCO

GOVERNMENT

PRECIOUS METALS

WINE & SPIRITS

AUTO LUBRICANTS

OTHER INDUSTRIES
Our digital invisible technologies for product authentication and counterfeit protection can be applied to a broad range of branded products across a multitude of industries, including household appliances, electromechanical parts (e.g., circuit breakers), agrochemicals (e.g., pesticides), cosmetics, oil & gas (e.g., lubricants), consumer goods, food & beverage and more.
Would you like to see how this technology can be applied to your product?
Contact us!